Parallel detection of estrogenic and androgenic activity
One research topic at the department of Biochemistry and Ecotoxicology of the German BfG (Federal Institute of Hydrology) is the development and improvement of effect-based methods for the rapid and cost-effective screening of environmental samples. Researchers of the Life Sciences Institute at the Hebrew University of Jerusalem focus on the genetic engineering of whole-cell biosensors, the development of novel bioreporters and the directed evolution process for the improvement of sensor performance. The collaboration between these two working groups is part of the German-Israeli cooperation in the water technology research project „Tracking Effects of Environmental organic micro-pollutants in the Subsurface“ (TREES), aiming at broadening the application of bioassays performed in combination with HPTLC.
Introduction
Endocrine disrupting compounds that enter the aquatic environment, e.g., via wastewater treatment plant (WWTP) effluents, can affect the natural hormonal activity of freshwater organisms and thus pose a potential ecological threat even at low concentrations [1]. Prominent endocrine disrupting compounds bind to estrogen (ER) and androgen (AR) receptors, and thus trigger estrogenic and androgenic activity. These modes of action are detected with genetically engineered yeast cells, emitting a detectable fluorescence signal upon exposure to compounds exerting hormonal activity.
Following studies performed with Arxula adeninivorans yeast strains [2], the authors show here the parallel biological detection of estrogenicity and androgenicity in HPTLC-separated sample components using newly generated biosensors based on Saccharomyces cerevisiae. These biosensors express human estrogen and androgen receptors, co-transfected with respective reporting elements that produce either the red fluorescent protein mRuby2 in response to an estrogenic stimulus (ER-Ruby) or the blue fluorescent protein mTagBFP2 in response to an androgenic stimulus (AR-BFP).
The advantage of using HPTLC lies in its biocompatibility, which allows the combination of sample fractionation with biological effect detection directly on HPTLC plates. Several samples can be screened for estrogenicity and androgenicity on parallel tracks simultaneously, thus reducing time and effort. Its matrix robustness allows the assessment of samples with complex and heavy matrices such as WWTP influents.
Standard solutions
Stock solutions of testosterone (0.5 mg/mL) and dihydrotestosterone (DHT, 5 mg/mL) are prepared in ethanol as androgenic reference compounds. Estrone (E1), 17ß-estradiol (E2) and estriol (E3) in ethanolic stock solutions of 5 mg/mL serve as estrogenic reference compounds. An androgenic and an estrogenic mixture was prepared and diluted depending on range-finding tests.
Sample preparation
Influent and effluent grab samples of two different municipal WWTP are enriched 200x and 500x by solid-phase extraction, respectively.
Chromatogram layer
HPTLC plates silica gel 60 F254 (Merck), 20 x 10 cm, are used. Plates are pre-developed with 100% methanol to 95 mm, dried in an oven at 120 °C, and then stored in a desiccator at room temperature.
Sample application
The application of samples and standard solutions as bands is performed with the Automatic TLC Sampler (ATS 4): up to 11 tracks, band length 5.0 mm, distance from left edge 20.0 mm, track distance 16.0 mm, distance from lower edge 8.0 mm, application volume 10.0 to 20.0 μL for sample solutions and 5.0 to 10.0 μL for standard solutions.
Chromatography
HPTLC plates are developed in the Automated Multiple Development System (AMD 2). A focusing step is performed with 100% methanol to a migration distance of 20 mm, followed by drying for 2 min. In a second step, the development to a migration distance of 90 mm is conducted with ethyl acetate – n-hexane 1:1 (v/v) followed by drying for 5 min.
Yeast biosensors
For the planar bioassay, a yeast co-culture is prepared by mixing AR-BFP and ER-Ruby yeast cell cultures, previously adjusted separately to 1000 ± 50 formazine attenuation units. Then, 3.0 mL of this yeast co-culture are sprayed onto HPTLC plates using the Derivatizer (nozzle: yellow, level: 5). Plates are incubated at 30 °C for 18 h.
Documentation
Images are captured in UV 366 nm and white light with the TLC Visualizer.
Densitometry
Fluorescence measurement is performed with TLC Scanner 4 and visionCATS (slit dimension 10.0 x 0.6 mm and scanning speed 20 mm/s). The slit-dimensions reported in Schoenborn et al. (2017) [3] were used for these experiments; however, a further optimization of the slit dimensions under consideration of the signal broadening might improve the results. For detection of estrogenicity (ER-Ruby), excitation wavelength λex = 525 nm and a K540 nm filter is applied. The detection of androgenicity (AR-BFP) is performed using an excitation wavelength λex = 396 nm and a K400 nm filter.
Results and discussion
The aim of the presented study was to generate yeast-based fluorescent biosensors for the parallel detection of estrogenicity and androgenicity. In contrast to yeast-based biosensors using the expression of LacZ as reporting element, which requires the disruption of the cell wall and the addition of an external substrate [3], the presented biosensors allow a fast signal detection in living cells.
The general functionality of the individual yeast biosensors was assessed in 96-well plates and in combination with HPTLC, using either individual reference compounds or their mixtures [4].
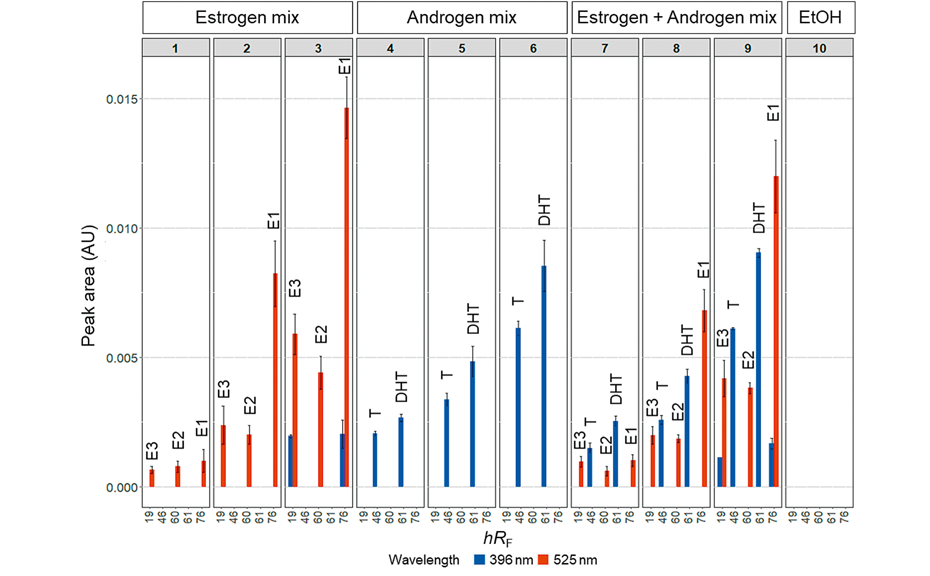
Then, the combination of the two yeast biosensors as co-culture was investigated, using three dilutions of an estrogen mix of E1, E2 and E3 and an androgen mix of testosterone and DHT. The estrogen mix was applied on tracks 1–3, the androgen mix on tracks 4–6, and both mixtures were applied on tracks 7–9. Ethanol served as control on track 10. A distinction of the different hormonal activities in the same test can be made by changing the excitation wavelength for scanning. The repeatability was determined by calculating the standard error of minimum three replicates performed on different days, using separate aliquots of the cell suspension.
Dose-effect relationships of the estrogenic and androgenic components in the mixtures were detected. The ER-Ruby biosensor detects only estrogenic compounds (red), even in the presence of androgenic compounds, highlighting the specificity of this biosensor. A slight decrease in the signal intensity at λex = 525 nm was detected, when estrogenic and androgenic model compounds were applied in a mixture. This suggests a weaker expression of the Ruby protein under these conditions.
Regarding the AR-BFP strain, the signal intensity of androgenic compounds (blue) remains unchanged in the presence of estrogenic compounds. However, some interference at the hRF values specific for E1 and E3 is observed in case of high concentrations of the reference compounds. These signals were also detectable when only the ER Ruby biosensor was applied [4]. Possibly, high concentrations of the Ruby protein lead to detectable excitation of Ruby at λex = 396 nm. On the other hand, the used lamps have a low output at 396 nm, which is as well very close to the cut-off filter at 400 nm. In any case, the optical system has to be refined by optimized filters used for the scanning, which might help avoiding this artefact in the future.
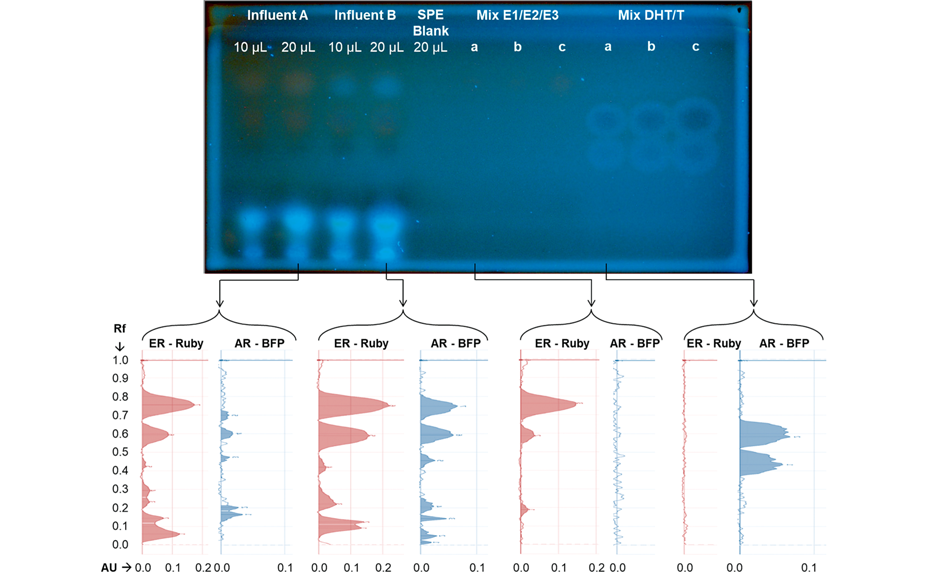
An example of the yeast-based biosensor application for a simultaneous screening for androgenicity and estrogenicity using influent samples from a municipal WWTP is shown below. Matrix components are observable below the focusing line and are thus well separated from active compounds. Both influent samples showed estrogenicity and androgenicity. Due to the similar structures and physico-chemical properties of estrogens and androgens, a separation of the two compound classes by HPTLC is challenging [2]. However, the multi-parallel effect assessment in combination with HPTLC allows the detection of the different effects even if the active compounds are not fully separated. In the presented WWTP influents, two strong estrogenic and androgenic signals overlap at hRF values of 75 and 61, indicating the possible presence of E1 (hRF = 76), E2 (hRF = 60) and DHT (hRF = 61). For the androgenic band at hRF = 75, no candidate compound can be assigned. At some positions, signals from the ER-Ruby strain are visible between the tracks. Due to the leakiness of promoters, each reporter strain produces a background signal, which might be detectable especially at positions where cell densities are higher due to an inhomogeneous application of cells. However, an automated spraying device (Derivatizer) was used resulting in an even application of the cells. A further possibility is a signal broadening that can happen when silicaplates are used.
In conclusion, the presented study showed a proof of principle of the parallel detection of estrogenic and androgenic effects using a fluorescent yeast-based biosensor co-culture.
-
01
Applicability of a yeast co-culture for parallel detection of estrogenicity and androgenicity in model compound mixtures by fluorescence scanning at λex = 396 nm (blue) and λex = 525 nm (red). Estrogen mix consisting of E1 (0.01 ng, 0.05 ng and 0.1 ng), E2 (0.005 ng, 0.01 ng and 0.02 ng), and E3 (0.5 ng, 1 ng and 2 ng) was applied on tracks 1–3 and 7–9. Androgen mix consisting of testosterone (T; 0.5 ng, 1.0 ng and 5.0 ng) and DHT (0.5 ng, 1.0 ng and 5.0 ng) was applied on tracks 4–6 and 7–9. Reproduced with modifications from [4] (https://creativecommons.org/licenses/by/4.0/legalcode ).
-
02
Parallel detection of estrogenicity and androgenicity in wastewater treatment plant influents using a yeast-based biosensor co-culture of ER-Ruby (red, λex = 525 nm) and AR-BFP (blue, λex = 396 nm). Estrogen mix: E1 (a: 0.1, b: 0.2 and c: 0.4 ng), E2 (0, 20 and 40 pg) and E3 (1, 2 and 4 ng); androgen mix: DHT (a: 10, b: 25 and c: 50 ng) and testosterone (T; a: 10, b: 25 and c: 50 ng). Top: plate image displaying the fluorescent signal enhanced using the enhancement tool of the visionCATS software. Reproduced with modifications from [4] (https://creativecommons.org/licenses/by/4.0/legalcode).
Literature
[1] A.P.A. Da Silva et al. Aquat. Sci. Technol (2018) 6: 35-51.
[2] A. Chamas et al. Sci. Total Environ. (2017) 605–606:507–513.
[3] A. Schoenborn et al. J Chromatogr A (2017) 1530:185–91.
[4] L. Moscovici et al. Biosensors (2020) 10(11): 169.
Contact:
Carolin Riegraf, Federal Institute of Hydrology, Am Mainzer Tor 1, 56068 Koblenz, Germany. Present address: Swiss Centre for Applied Ecotoxicology, Überlandstrasse 133, 8600 Dübendorf, Switzerland, carolin.riegraf[at]oekotoxzentrum.ch
The authors acknowledge Marina Ohlig and Ramona Pfänder also involved in the TREESproject funded by the Federal Ministry of Education and Research (BMBF) Germany (Grant No. 02WIL1387), and the Ministry of Science, Technology and Space (MOST) Israel (Grant No. WT1402/2560).