HPTLC for quality differentiation of functional mushrooms
Nammex specializes in the production of high-quality, certified organic mushroom extract powders for the food and dietary supplement (DS) industries. As a result of the rapid growth of the functional mushroom market, we have observed the introduction of many new products of varying quality. Nammex has a long-standing history of leading the industry in product analysis, with a focus on ensuring product authenticity and efficacy [1]. Our laboratory has developed an innovative HPTLC method for the identification and quality control testing of diverse species used in DS products. With this method, we aim to enhance the overall reliability and transparency of quality testing in the industry.
Introduction
The functional mushroom market is experiencing significant growth, driven by factors like increased DS usage and ongoing medical research. Despite the market’s size, only one validated HPTLC mushroom identification method has been published (USP Ganoderma lucidum monograph) [2], and its indiscriminate use across other species may lead to misidentification, undermining the reliability of the identification process and creating a need for more comprehensive testing solutions.
HPTLC is widely recognized for its effectiveness in botanical identification, making it an ideal method for mushroom analysis. In the absence of validated methods, consumers risk exposure to mislabeled or adulterated products. For instance, products containing tempeh-like mycelium (i.e. vegetative body) fermented grain are often marketed as mushrooms (i.e. fruiting bodies) despite significant compositional differences. Additionally, concentrated mushroom extracts may be deficient in specific marker compounds due to processing conditions.
HPTLC offers a robust, highly selective approach for mushroom differentiation. This new method ensures that characteristic compounds from diverse chemical classes in mushrooms are clearly separated, supporting accurate species identification. The advantages of HPTLC in this context include its specificity, versatility, and ability to detect adulteration in complex products.
Standard solutions
Standard stock solutions are prepared at 0.5 mg/mL in methanol.
Sample preparation
Samples consist of 250 mg of mushroom extract powder or finely milled whole mushrooms. These are extracted in 5.0 mL of methanol, vortexed for 10 s, sonicated for 10 min at room temperature, and centrifuged at 3500 rpm for 10 min. The supernatant is then transferred to vials.
Chromatogram layer
HPTLC plates silica gel 60 F254 Premium Purity (Supelco, Merck), 20 × 10 cm are used.
Sample application
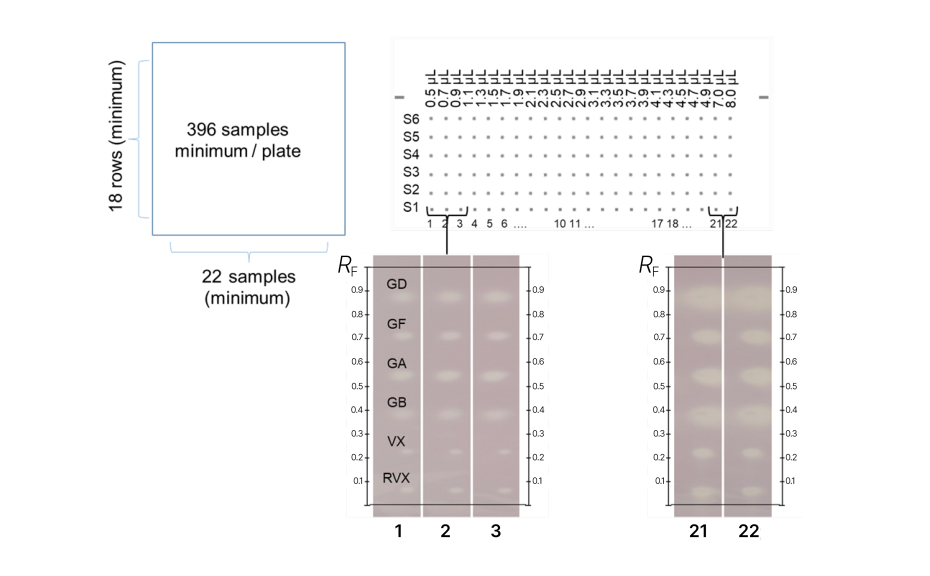
10.0 μL of sample solutions and 2.0 μL of standard solutions are applied as bands with the Automatic TLC Sampler (ATS 4), 15 tracks, band length 8.0 mm, distance from the left edge 20.0 mm, track distance 11.4 mm, distance from the lower edge 8.0 mm.
Chromatography
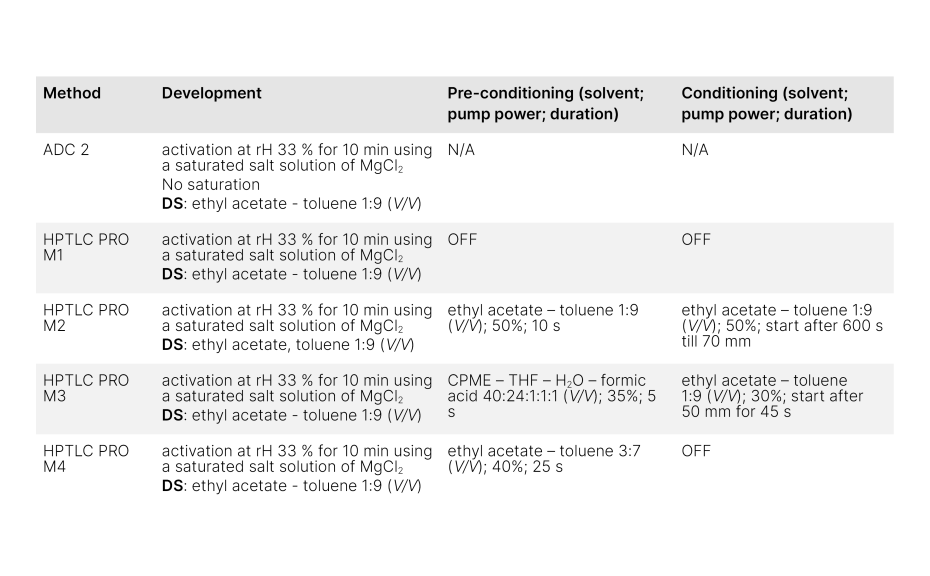
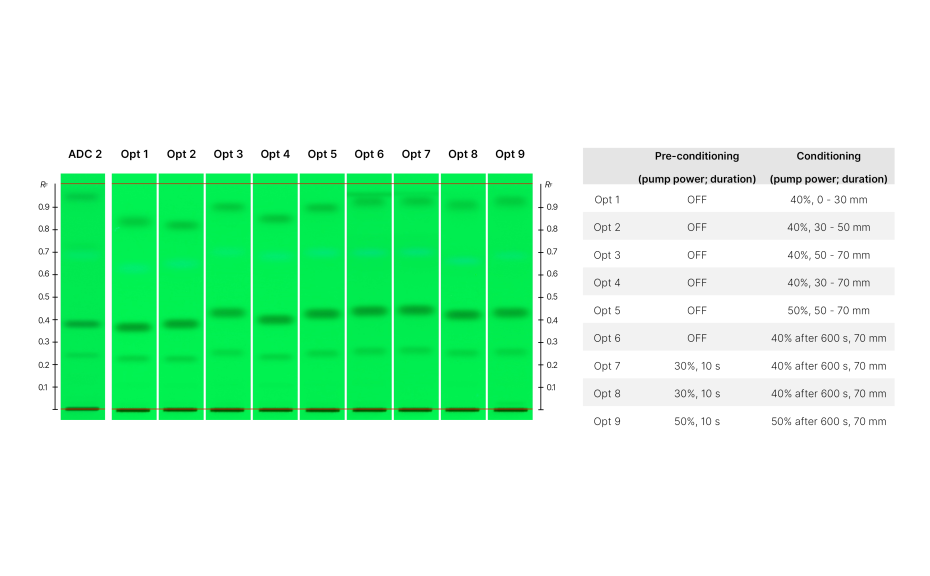
Plates are developed in the ADC 2, with chamber saturation (with filter paper) for 20 min and after activation at 33 % relative humidity for 10 min using a saturated magnesium chloride solution, development with toluene – methanol – and acetic acid 85:10:5 (V/V) to the migration distance of 70 mm (from the lower edge), followed by drying for 5 min.
Post-chromatographic derivatization
The plates are immersed into p-anisaldehyde sulfuric acid reagent using the Chromatogram Immersion Device (immersion speed: 5 cm/s, immersion time: 0 s). After derivatization, the plates are heated at 100 °C for 4 min using the TLC Plate Heater.
Documentation
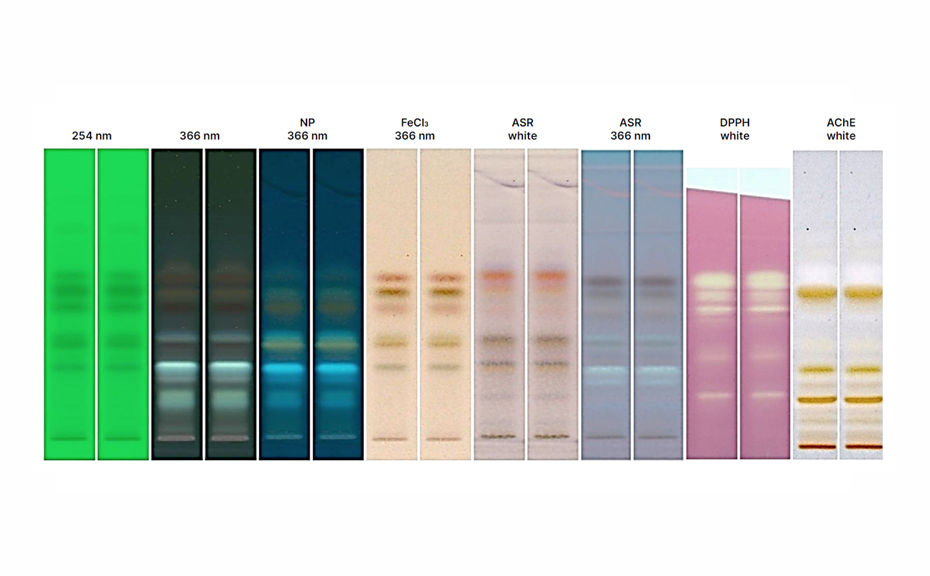
Images of the plates are captured with the TLC Visualizer 3 in UV 254 nm, UV 366 nm, and white light after development, and again after derivatization in UV 366 nm and white light.
Results and discussion
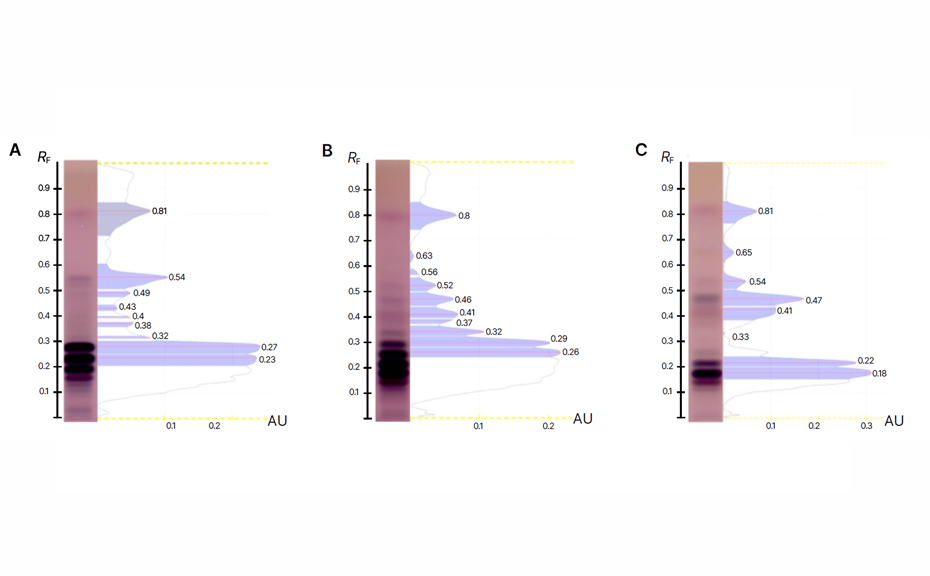
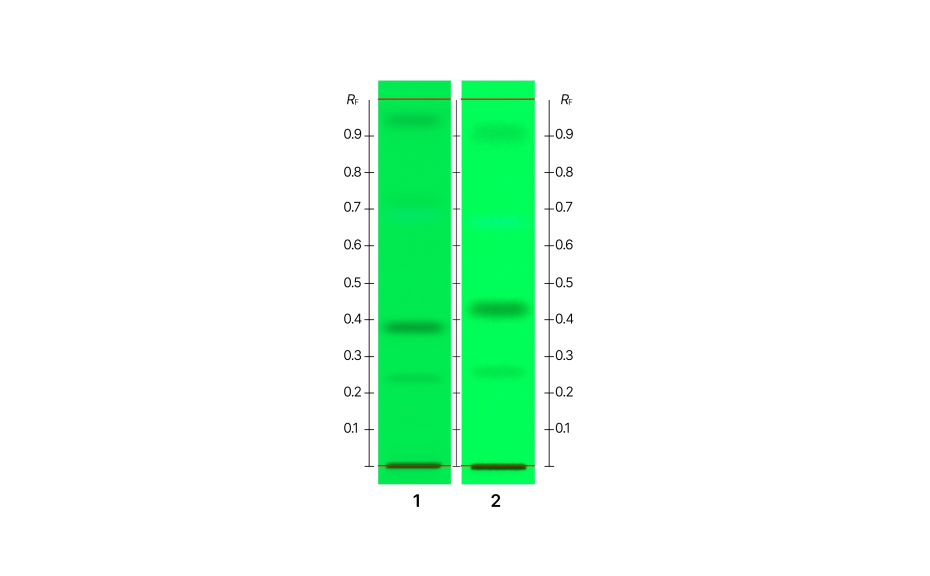
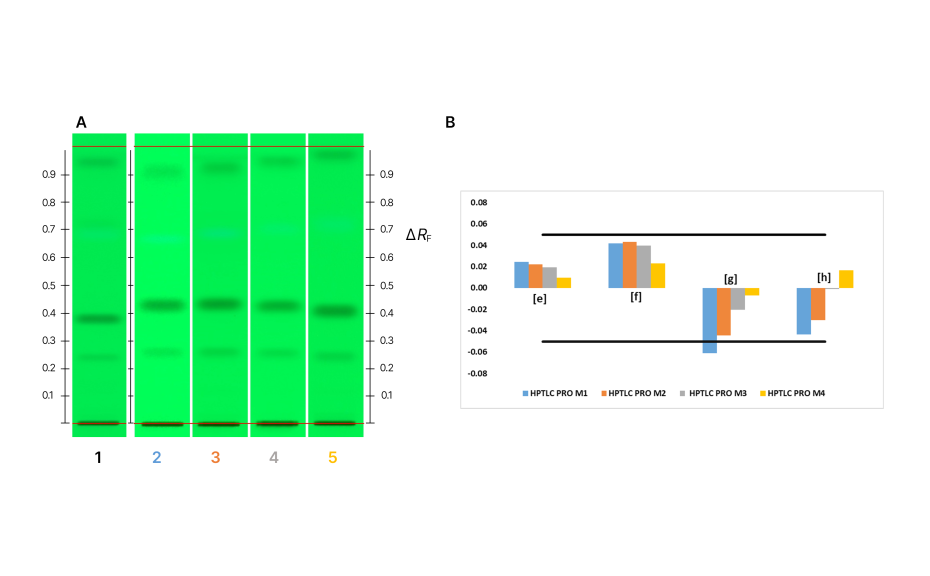
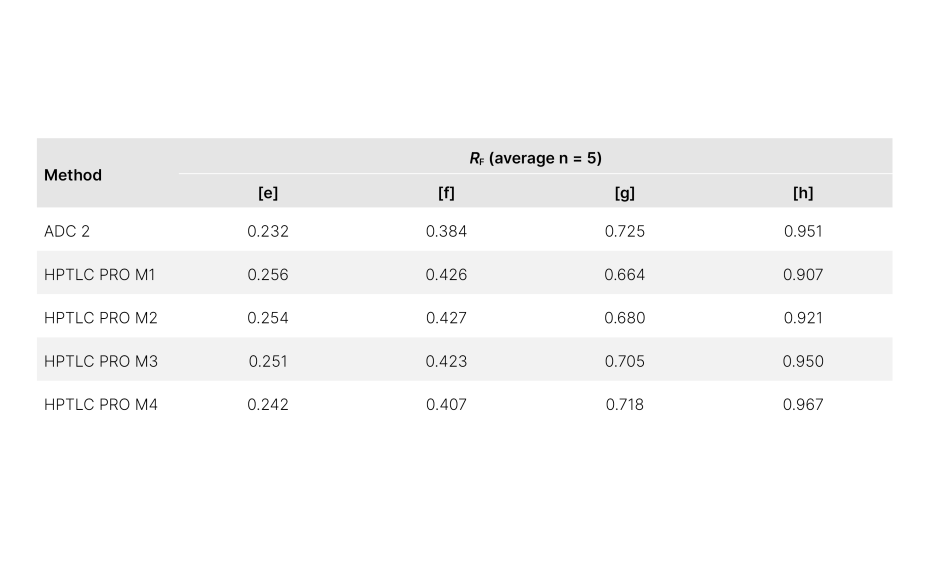
The high selectivity of the HPTLC method is demonstrated through distinct chromatographic fingerprints obtained for each species. These fingerprints display characteristic bands under multiple detection modes, providing a reliable means of differentiating between species and product types such as mushroom extracts and mycelia fermented grain powders.
Key marker compounds for each species were identified through literature, playing a critical role in distinguishing between the mushroom and the mycelium. Specifically, the mushroom is known to exhibit a different profile of compounds than the mycelium. HPTLC comparisons of mushroom extracts, supported by these chemical markers, effectively demonstrate these differences.
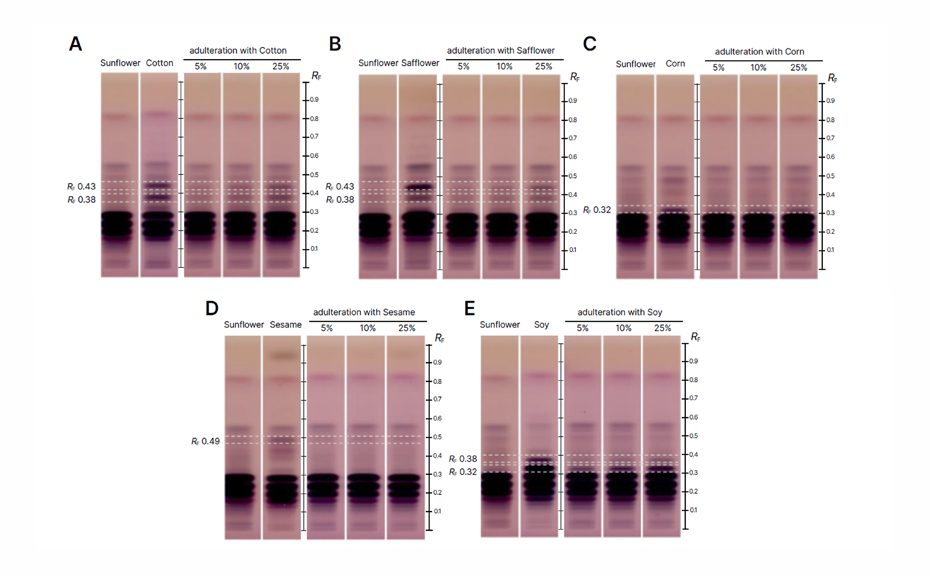
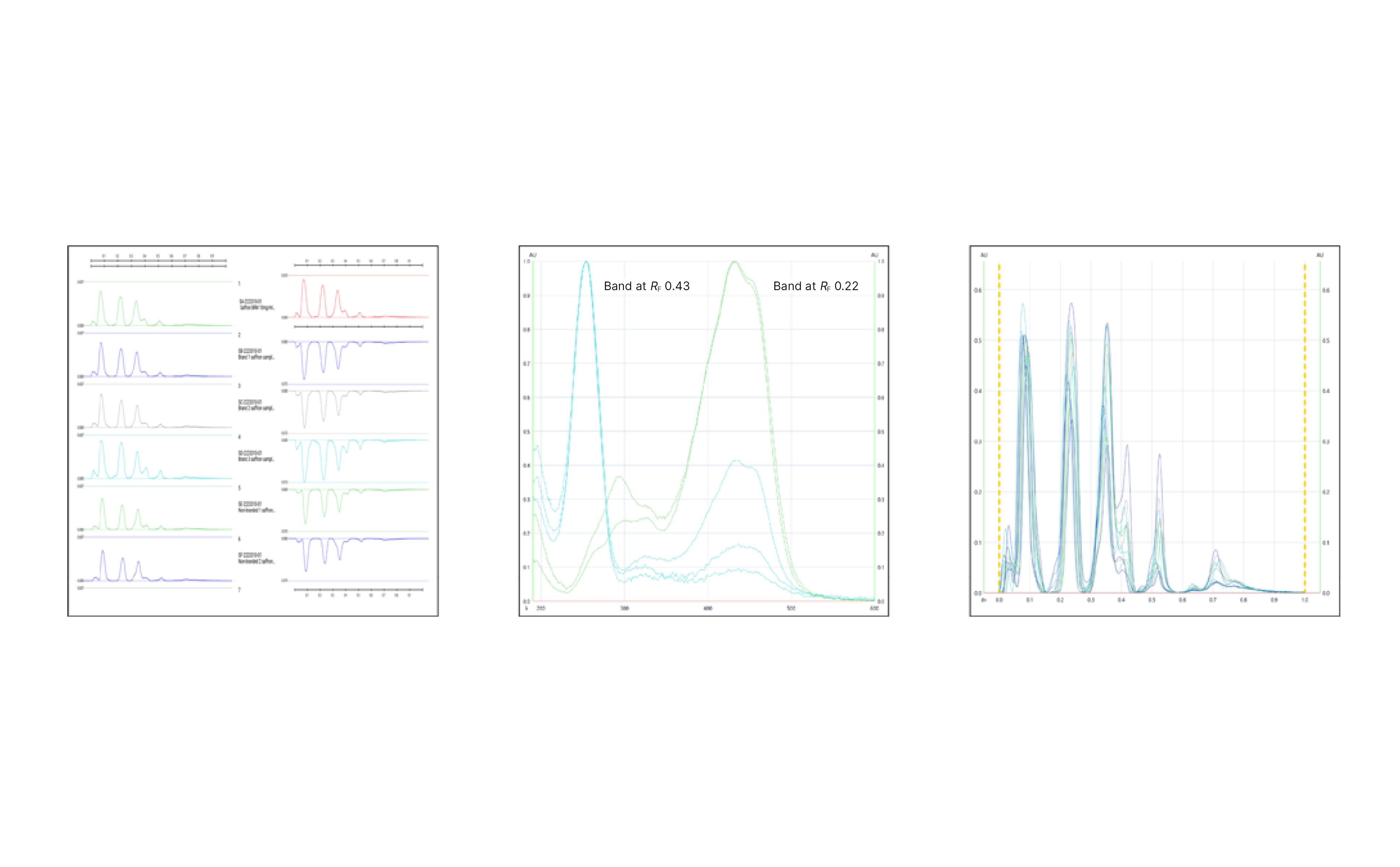
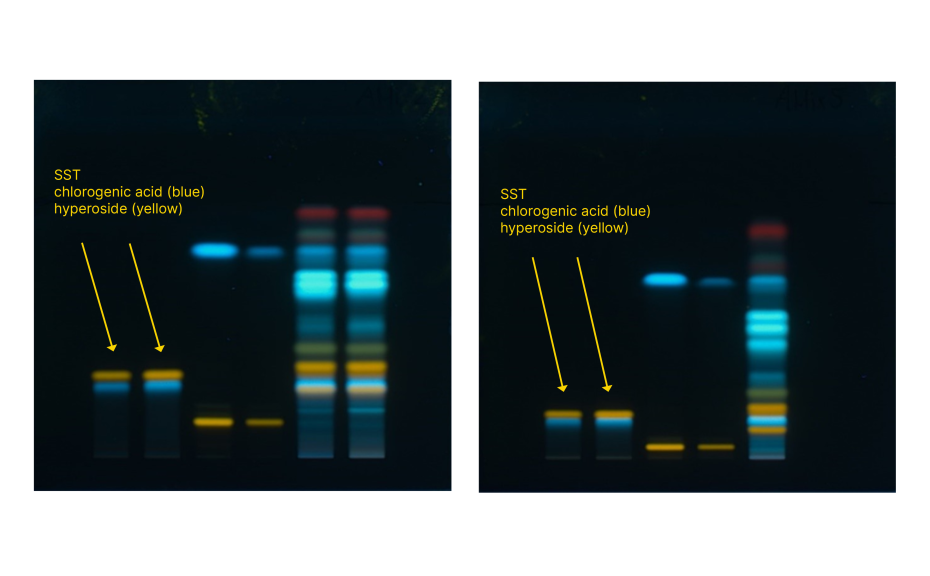
HPTLC comparisons between Chaga conk, pure mycelium, and fermented grain forms reveal significant compositional differences, with fermented grain fingerprints closely matching grain reference materials. Importantly, Chaga triterpenoid markers are absent in fermented grain, which instead shows high concentrations of triglycerides and linoleic acid. These chromatograms highlight the clear differences between Chaga conk, 1:1 extract, brown rice and oats, and fermented grain products, underscoring HPTLC’s effectiveness in detecting potential adulteration and verifying product authenticity.
While fermented grain products are expected to contain grain, the lack of sufficient mycelium or relevant compounds, along with unclear labeling practices, raises concerns about product authenticity. Many fermented grain products prominently display “mushroom” on the front label, along with images of mushrooms, but only disclose their myceliated grain content on the back, with some brands failing to identify the grain entirely. This inconsistency in labeling, coupled with the compositional differences identified through HPTLC, underscores the urgent need for more transparent and stringent quality control measures in the mushroom supplement industry.
-
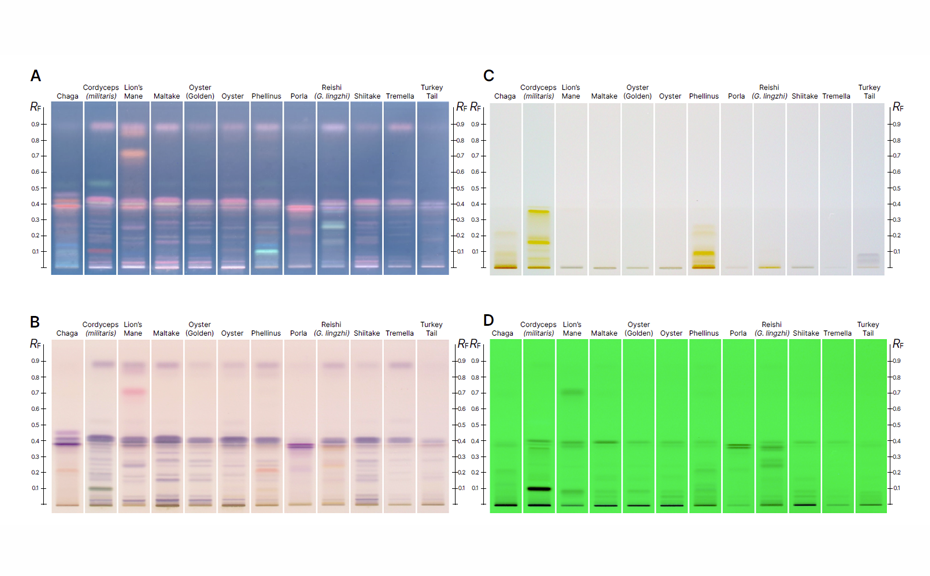
01
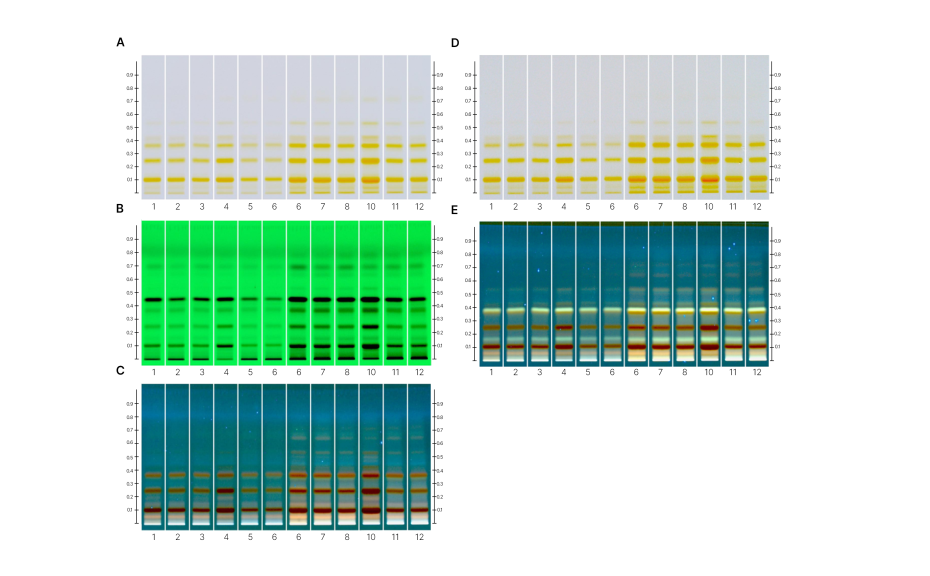
HPTLC chromatograms of whole mushroom, conk, or sclerotium vouchers from 12 species, highlighting compositional differences between species under various detection modes. Images after derivatization are shown in UV 366 nm (A) and white light (B). Chromatograms captured after development are displayed in white light (C) and 254 nm UV light (D).
-
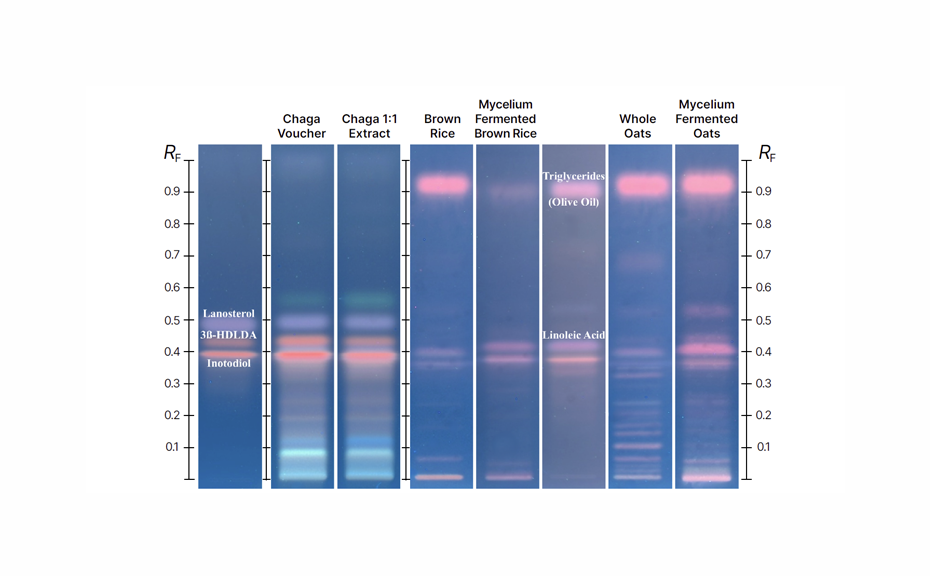
02
HPTLC comparisons between Chaga conk voucher and fermented grain forms reveal significant compositional differences, with fermented grain fingerprints closely matching grain reference materials. Key Chaga marker compounds – such as inotodiol (
In conclusion, the development of the innovative HPTLC method for the differentiation of functional mushrooms offers a significant advancement in ensuring product authenticity and quality within the growing mushroom supplement market. By providing clear, reliable chromatographic fingerprints for various species, this method enhances the ability to detect adulteration and verify product composition, particularly in distinguishing between mycelia fermented grain-based products marketed as mushrooms. As the market continues to expand, the implementation of robust, transparent quality control measures like HPTLC will be critical in maintaining consumer trust and safeguarding product efficacy.
Literature
[1] Chilton, Jeff. White Paper. Redefining Medicinal Mushrooms: A New Scientific Screening Program for Active Compounds. Nammex, 2015. jeff@nammex.com
[2] United States Pharmacopeia (USP). Ganoderma lucidum Fruiting Body Monograph. USP 43-NF 38, United States Pharmacopeial Convention, Rockville, MD, 2020.
Contact: Coleton Windsor, Nammex, Box 1780, Gibsons, British Columbia, Canada, coleton@nammex.com