
Tire particles assessed with HPTLC bioassays
Alan Bergmann, Benoit Ferrari, and Etienne Vermeirssen at the Ecotox Centre (Dübendorf, Switzerland), Thibault Masset and Florian Breider at EPFL (École Polytechnique Fédérale, Lausanne, Switzerland), and William Dudefoi (currently Aquatox Solutions) and Kristin Schirmer at Eawag (Dübendorf, Switzerland). The group is funded by the World Business Council for Sustainable Development’s (WBCSD) Tire Industry Project (TIP) to investigate the source and mechanisms of biological effects of chemicals from tire particles, the accumulation and trophic transfer of chemicals and particles, and potential solutions. WBCSD’s TIP is a global chief executive officer–led initiative undertaken by leading tire manufacturing companies that drives research on potential human health and environmental impacts of tires throughout their life cycle. The study design, execution, interpretation, and manuscript preparation were conducted solely by the authors.
Introduction
Tire and road wear particles (TRWP) are formed by abrasion of tires with road surface. They currently make up a large proportion of primary microplastics released to the environment [1]. TRWP are of particular concern because of many unbound chemical additives of tire rubber and potential unknown impurities and reaction products, all of which may leach from the particles into the environment [2]. We applied HPTLC bioassays for estrogenicity (YES), genotoxicity (umuC), and bacterial luminescence inhibition (BLIT) to evaluate the toxicity of chemicals extracted and leached from lab-generated cryogenically-milled tire tread (CMTT).
HPTLC-bioassays are useful for (1) sensitive detection of biological activity compared to testing the whole mixture in microtiter plates, (2) revealing differences in the profile of toxicity (i.e. differences in the bands between samples, even if the total toxicity is similar), and (3) comparing to individual standard chemicals to include or exclude chemical candidates as potentially responsible for the toxicity. HPTLC-bioassays are limited in the quantitative assessment of total sample toxicity because of greater variability than microtiter versions. This, and our focus on detecting and identifying hazardous chemicals in higher-than-environmentally relevant concentrations of CMTT, mean our work is a qualitative evaluation of worse case scenarios.
Standard solutions
Eleven tire‐associated chemicals were purchased and prepared in methanol or acetone at 0.5 to 1 g/L. Positive controls for the bioassays (YES: 0.80 pg 17β-estradiol, umuC: 0.31 and 2.5 ng 4-nitroquinoline-N-oxide, BLIT: 630 ng caffeine and 65 ng 3,5-dichlorophenol) are applied as solutions in ethanol, acetone, or methanol, respectively.
Sample preparation
Tire tread is cut from new tires from three manufacturers then cryogenically milled into small particles (CMTT) [3]. Soxhlet extracts in dichloromethane and methanol, leachates into simulated fish digestion fluid, and elutriates into water and artificial sediment are created to 100 g/L CMTT. Leachates are further processed with liquid-liquid extraction to ethyl acetate:n-hexane 9:1 (V/V).
Chromatogram layer
HPTLC plates silica gel 60 (Merck), 20 × 10 cm area used.
Sample application
Up to 50.0 µL of sample solutions and 5.0 µL of standard solutions are applied as bands with the Automated TLC Sampler 4 (ATS 4), band length 6.0 mm, distance from the left edge 20.0 mm, track distance 12.0 mm, distance from the lower edge 10.0 mm.
Chromatography
For initial screening of samples in all bioassays, development with the Automated Multiple Development 2 (AMD 2): twice with methanol to the migration distance of 20.0‐mm, acetone to 40.0 mm, acetone – ethyl acetate 3:1 (V/V) to 50.0 mm, ethyl acetate to 60.0 mm, ethyl acetate – n‐hexane 2:1 (V/V) to 70.0 mm, ethyl acetate – n‐hexane 1:1 (V/V) to 80.0 mm. Atmospheric conditioning solution was 10.0 mL 25 % NH4OH in 200.0 mL distilled deionized water. After an initial screening, the AMD 2 was adjusted to improve separation of estrogenic signals clustered near the solvent front: twice with methanol to 20.0 mm, methanol – ethyl acetate 1:1 (V/V) to 40.0 mm, ethyl acetate – n‐hexane 1:1 (V/V) to 60.0 mm, ethyl acetate – n‐hexane 1:9 (V/V) to 80.0 mm.
Post-chromatographic derivatization
Yeast (YES) or bacteria (umuC and BLIT) suspensions were sprayed with the Derivatizer (red nozzle, level 6). For YES and umuC, a solution of 4-methylumberifferylgalactopyranoside (MUG) was sprayed after 3 or 2 hours of incubation at 30 or 37 °C, respectively.
Documentation
Responses of YES and umuC were documented with the Visualizer 2 in UV 366 nm.
Luminescence from the BLIT was recorded immediately after spraying bacteria with the Bioluminizer using 1 min exposures.
Densitometry
Optional: Fluorescence measurement (for umuC and YES) is performed with the TLC Scanner 4 and visionCATS at 366>/400 nm (mercury lamp), slit dimension 5.00 mm x 0.20 mm, scanning speed 20 mm/s.
Results and discussion
An image of tire extracts, digestates, and elutriates tested with the HPTLC-YES is shown. Estrogenic chemicals are present in all extracts and leachates of CMTT. Similar retention factors between some bands in the samples suggest that similar chemicals are responsible for estrogenicity, however the relative intensity of the bands varies.
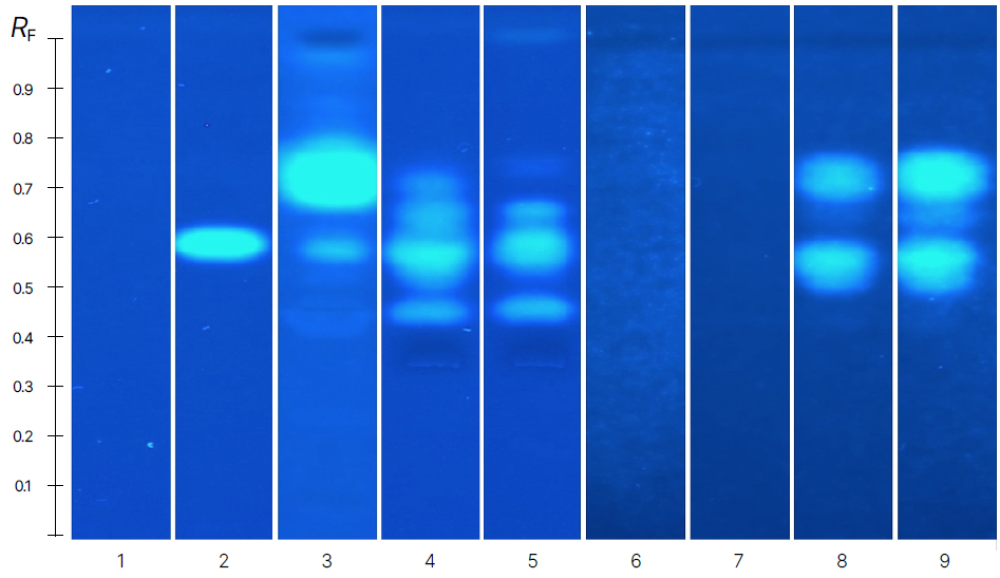
High-performance thin-layer chromatography (HPTLC) bioassay images of tire particle extracts, digestates, and water/sediment leachates. (A) Yeast estrogen screen (YES), positive control: 17β-estradiol 4 pg. (B) umuC, positive control: 4-nitroquinoline-N-oxide 2.5 ng. (C) Bacterial luminescence inhibition test (BLIT), positive control: caffeine 625 ng. Cryogenically milled tire tread (CMTT) Soxhlet extract at 0.5 mg CMTT equivalent (YES), 1 mg (umuC), and 0.5 mg (BLIT). Aqueous samples (digestates, water, and sediment leachates and corresponding process controls) were extracted with liquid–liquid extraction into ethyl acetate:n-hexane prior to HPTLC bioassay testing and applied at 5 mg CMTT equivalent. Reprinted from Creative Commons Attribution 4.0 International [4].
A blank sample, representing background chemicals from the simulated digestive fluid was also estrogenic. The toxicity profile of the blank digestate shows which bands in the CMTT digestate originate from digestion materials, not CMTT. One band in the CMTT digestate at about RF = 0.7 is likely from the CMTT, and more may remain co-retained with the digestate background estrogens. We linked the blank estrogenicity to biologically sourced components of the digestates: porcine bile and pancreatin. HPTLC allowed us to distinguish some of this background from chemicals coming from CMTT. There may be additional CMTT chemicals obscured by the digestates background estrogenicity.

HPTLC-YES of artificial digestate and its components. HEPES (0.04 M), pancreatin (4 mg/mL in saline solution), porcine bile extract (10 mg/mL in electrolyte solution), and pepsin (5 mg/mL in 0.25 M HCl), were prepared individually approximately following Masset et al. Pancreatin, Porcine bile extract, and pepsin were passed through 0.45 ul PTFE filters to remove slight precipitation, then processed with LLE as described in the main text. Fifty uL of the extracts were applied to the HPTLC plates. Reprinted from Creative Commons Attribution 4.0 International [4].
Ten of eleven chemicals were active in at least one bioassay. Some of these chemicals had similar retention factors to bioactive bands from the CMTT samples. For example, some benzothiazoles and 6PPD were retained in the region RF 0.7-0.85. These results help implicate benzothiazoles as drivers of in vitro toxicity of CMTT to bacteria. However, confirmation of individual benzothiazoles as the responsible toxicants would be needed, possibly through chemical analysis of the active CMTT bands. Subsequently, any risk to organisms in the environment requires evaluation of environmentally relevant concentrations and quantitative toxicological analysis. Our work is an early step in the risk assessment process by expanding the understanding of potential hazards from tire particles, and prioritizing chemicals as potentially responsible for their effects.
Chemicals detected in cryogenically milled tire tread (CMTT) extracts and their bioactivity in high‐performance thin‐layer chromatography (HPTLC) bioassays
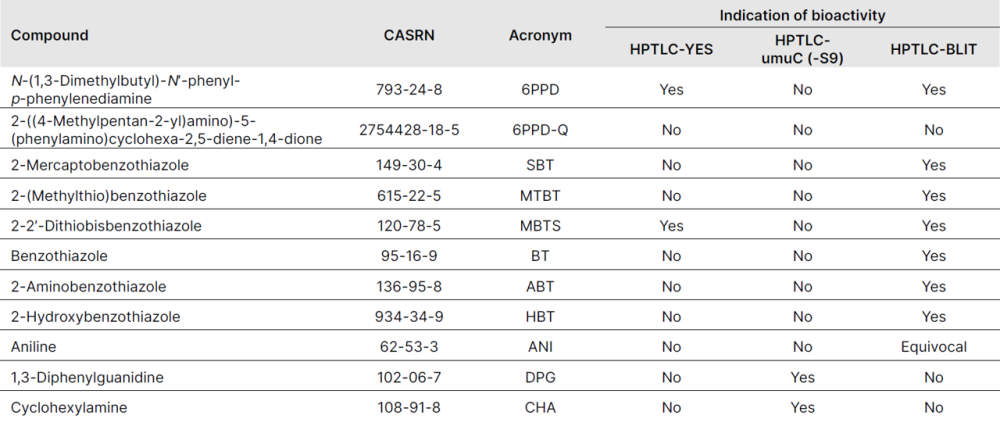
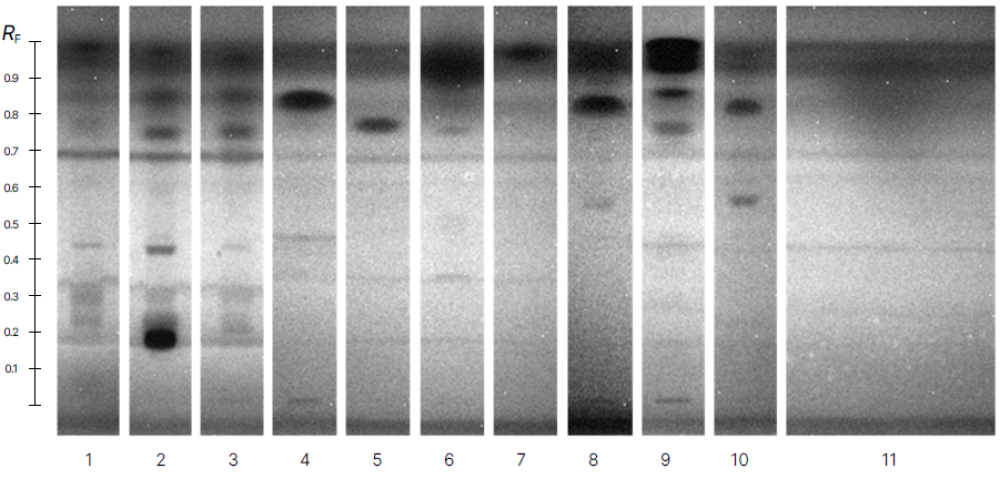
High-performance thin-layer chromatography–bacterial luminescence inhibition test of active single chemicals compared with cryogenically milled tire tread samples. Chemical amounts are nominally 0.55 μg (HBT), 0.50 μg (ABT), 5.15 μg (BT), 0.5 μg (MTBT), 0.66 μg (SBT), 0.7 μg (6PPD), 0.14 μg (MBTS), and 4.2 μg (ANI). See Figure 1 for representative negative and positive controls. CMTT = cryogenically milled tire tread; HBT = 2-hydroxybenzothiazole; ABT = 2-aminobenzothiazole; BT = benzothiazole; MTBT = 2-(methylthio) benzothiazole; SBT = 2-mercaptobenzothiazole; 6PPD = N-(1,3-dimethylbutyl)-N′-phenyl-p-phenylenediamine; MBTS = 2-2′-dithiobisbenzothiazole; ANI = aniline. Reprinted from Creative Commons Attribution 4.0 International [4].
Literature
[1] Sieber, R.; Kawecki, D.; Nowack, B., Dynamic probabilistic material flow analysis of rubber release from tires into the environment. Environmental Pollution 2020, 258, 113573.
[2] Wik, A.; Dave, G., Occurrence and effects of tire wear particles in the environment – A critical review and an initial risk assessment. Environmental Pollution 2009, 157 (1), 1-11.
[3] Masset, T.; Ferrari, B. J. D.; Dudefoi, W.; Schirmer, K.; Bergmann, A.; Vermeirssen, E.; Grandjean, D.; Harris, L. C.; Breider, F., Bioaccessibility of Organic Compounds Associated with Tire Particles Using a Fish In Vitro Digestive Model: Solubilization Kinetics and Effects of Food Coingestion. Environmental science & technology 2022, 56 (22), 15607-15616.
[4] Bergmann, A. J.; Masset, T.; Breider, F.; Dudefoi, W.; Schirmer, K.; Ferrari, B. J. D.; Vermeirssen, E. L. M., Estrogenic, Genotoxic, and Antibacterial Effects of Chemicals from Cryogenically Milled Tire Tread. Environ Toxicol Chem 2024, 43 (9), 1962-1972.
PDF Version of the article
Contact: Dr. Alan Bergmann, Swiss Centre for Applied Ecotoxicology, Dübendorf, Switzerland, alanjames.bergmann@oekotoxzentrum.ch