
Lipidomics of Thlaspi arvense seed maturation for biofuel production
The teams of Dr. Miguel Alfonso, molecular biologist and plant geneticist researcher at the Aula Dei Experimental Station (CSIC, Zaragoza, Spain), and that of Dr. Vicente L. Cebolla, analytical chemist researcher at the Instituto de Carboquímica (CSIC, Zaragoza, Spain), are collaborating in biofuel production from plant seeds, in particular from Thlaspi arvense, also known as pennycress. This winter plant is an emerging raw material for producing high-quality, erucic-rich biofuel. Pennycress has favorable agronomic properties, can be cultivated in arid and semi-arid drylands in cold climates, and does not compete with other crops that are edible, such as soybean or sunflower.
Introduction
The differential fact that gives pennycress seed oil extraordinary properties as a biofuel is the majority composition of their lipids in erucic acid, a mono-unsaturated, long-chain (C22), and other very long chain fatty acids. However, erucic acid is absent or barely present in oils from other related species (e.g., rapeseed, Arabidopsis thaliana) or in other used for biofuel production (soybean, Camelina sativa). To understand why, we set out to elucidate the molecular mechanisms of incorporation of erucic acid into TAG, in function of the maturation of the Pennycress seed. For this, we have carried out an exhaustive lipidomic study based on HPTLC coupled to MS, a choice technique for a rapid and informative lipidomic screening.
HPTLC-MS allowed us for obtaining normalized profiles of TAG subclasses. This was validated and statistically correlated using a standardized quantitative LC-MS based-method. Likewise, we were able to draw structural information about sn-2 position of certain TAG species by HPTLC-MS/MS. This was of importance in this research to determine which metabolic pathways of TAG biosynthesis are favored over others at different stages of seed maturation. This has been possible by crossing lipidomic results with those of an integrative transcriptomic analysis of gene expression (RNA-Seq and qPCR) [1,2]. The research is of interest for genetic improvement of other potentially biofuel-producing plants.
Standard solutions
Solutions of individual standards from a variety of lipid families (0.33-2 mg mL-1), including triacylglycerol (TAG) standards, were dissolved in DCM-MeOH (1:1, V/V) [1,2].
Sample preparation
For this study, five developmental stages of pennycress seeds were selected for lipidomic analysis, which correspond to green (G, 12 days after flowering, DAF), green-yellow (GY, 19 DAF), yellow-green (YG, 26 DAF), yellow (Y, 33 DAF), and mature (M, 45 DAF), according to a previous work [1]. They were harvested, frozen in liquid nitrogen, and stored at −80 °C.
Total lipids are extracted from seeds (0.1 g) with CHCl3-MeOH (6 mL, 2:1, V/V) using the “Bligh and Dyer” method. Samples are dissolved (3-4 mg mL-1) in DCM-MeOH (1:1, V/V).
Chromatogram layer and conditioning
HPTLC silica gel 60 plates (20 × 10 cm) without fluorescence indicators are employed (Merck). Before being used, plates are immersed in THF (5 min), dried at 70 °C in a vacuum (50 mbar) for 15 min, and pre-developed up to 90 mm using n-heptane (C7)-t-butyl-methyl-ether (MTBE)‒acetic acid (AcH), 70:30:1 (V/V).
Sample application
3 μL of standard solutions and 4 μL of sample solutions are applied as 4-mm bands in triplicate on the same plate, on three different plates with the Automatic TLC Sampler 4 (ATS 4), band length 6.0 mm, distance from the left edge 10.0 mm, minimal track distance 6.0 mm, distance from the lower edge 10.0 mm. One or more tracks are left empty, as blanks for chromatography and MS analysis.
Chromatography
The plates are developed in a horizontal developing chamber (20 × 10 cm) up to 70 mm using C7-t-butyl-methyl ether-AcH (70:30:1, V/V).
Densitometry
Detection was carried out using the TLC Scanner 3 at 190 nm in absorbance mode. WinCATS software was used to control and process data from sample application and densitometry.
Mass spectrometry
Zones at 52 mm migration distance were eluted with the TLC-MS Interface 2 (oval elution head) at a flow rate of 0.2 mL/min with methanol into an ion trap Amazon Speed Spectrometer (Bruker Daltonics, Bremen, Germany).
Electrospray ionization in positive ion mode, ESI(+)-MS, was selected for TAG analysis. MS spectra were recorded at the same ionization time in all cases. Structural identity of TAG was performed by ESI(+)-MS/MS.
Results and discussion
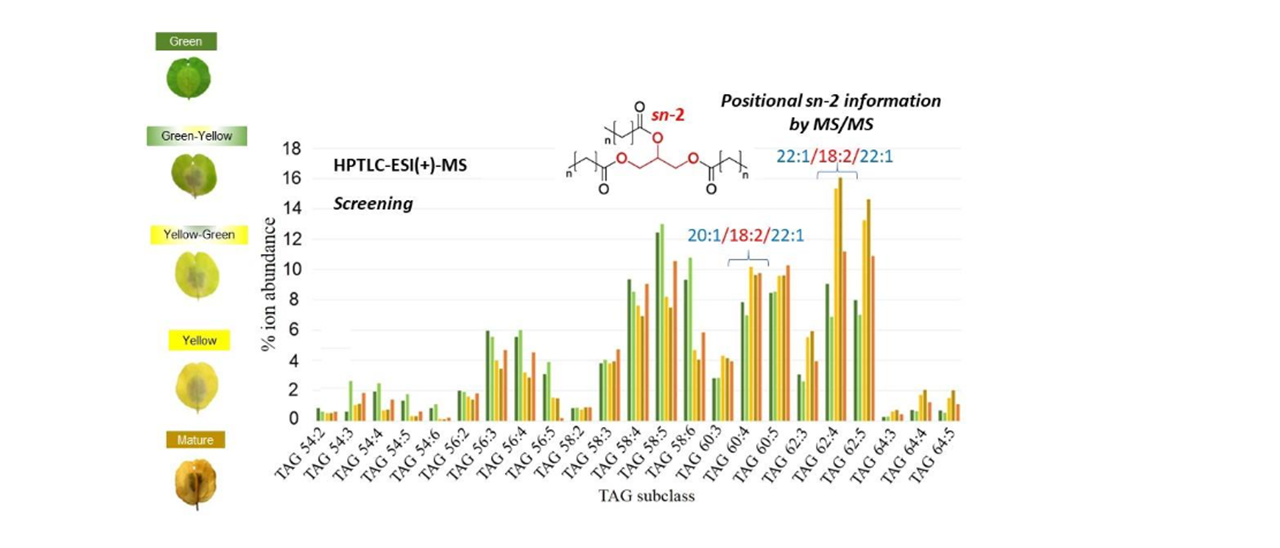
Densitograms show that TAG is the main peak in pennycress seeds (88-96% of the total peak area), increasing as a function of maturation, from the different stages from G to M. TAG bands, with a migration distance (m.d.) in HPTLC near to 52 mm, were sent to ESI(+)-MS in order to obtain normalized profiles of TAG ion abundance and the corresponding TAG subclasses, which provided similar results to those obtained by LC-MS [2]. In fact, results of both techniques were statistically correlated. Results confirmed the incorporation of 22:1 into TAG already at the initial stage of seed maturation (TAG 58:5), as well as the increase of 22:1 and 20:1 as maturation progresses (TAG 60:4 and TAG 62:4).
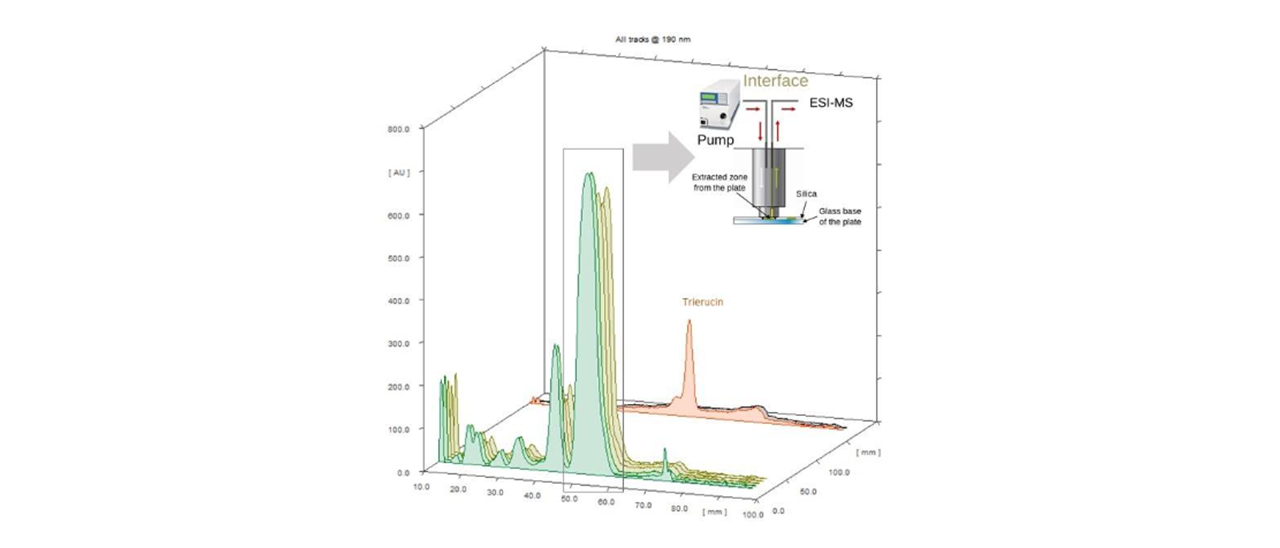
HPTLC densitograms of green stage pennycress seeds (two batches). Majority TAG band is extracted by the TLC-MS Interface 2 to an ion trap MS
The structural identification of TAG molecular subclasses was carried out by obtaining MS/MS spectra directly from the separated TAG band on the chromatographic plate by isolation and fragmentation of selected ions.
HPTLC densitograms of green stage pennycress seeds (two batches). The main TAG band is extracted using the interface to an ion trap MS.
A given TAG subclass usually consists of several isobaric species coming from different combinations of FA. This makes impossible a complete positional analysis, i.e., attribution to sn-1, sn-2, and sn-3 positions. However, there are occasions where only one combination of fatty acid composition is present. When a single triad of fatty acids is found in an MS/MS spectrum of a parent ion, we are able to identify the fatty acid in the sn-2 position. Why? It is known that existing fragmentation methods cannot distinguish between sn-1 and sn-3, as fatty acids from these positions in TAG have identical fragmentation efficiencies. Therefore, TAG molecules lose simultaneously and preferentially fatty acyls at positions sn-1 and sn-3. This implies the formation of two ions of similar abundance and more abundant than the ion corresponding to the loss of the fatty acid position at sn-2. Therefore, we can identify this one.
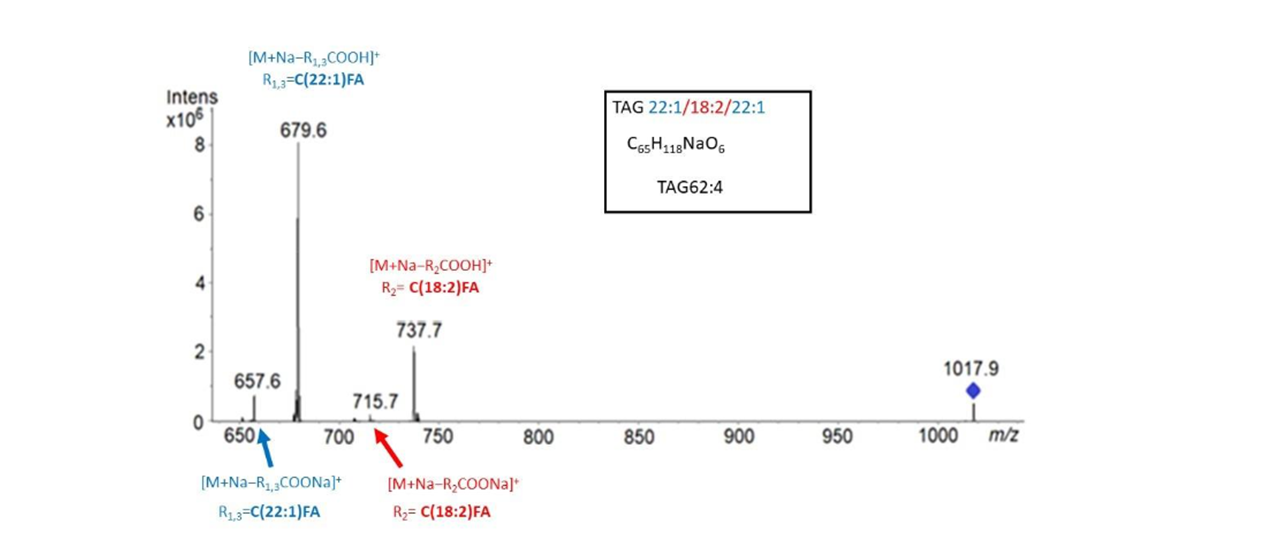
HPTLC-MS/MS spectrum of TAG (62:4) with detail of linoleic acid at sn-2
Positional analysis of acyl-groups at sn-1, sn-2, and sn-3 positions in TAG is important to analyze the influence of acyl-CoA pools and their incorporation into lipid species by the different TAG biosynthetic pathways. In general, it is assumed that acyl groups at the sn-1 and sn-3 positions of TAG are more related with the composition of the acyl-CoA pool, while those at sn-2 are directly related to the acyl preference of the lyso-phosphatidyl acyltransferase (LPAT) enzyme, which catalyzes sn-2 acylation in Kennedy’s pathway (de novo synthesis of TAG).
Our results indicated that in most of the major TAG species, 18:2 was detected at the sn-2 position in all stages of seed maturation, indicating that pennycress LPAT showed higher preference for 18:2-CoA substrates rather than for other CoA moieties such as erucoyl-CoA. In other words, this enzyme, does not show specificity for erucic acid.
Operationally, parent ion fragmentation in ion trap must be done in a limited period of time to obtain the corresponding MS/MS spectrum. Since multiple replicates of the same sample can be applied to the plate, and the chromatographic development is performed simultaneously for all applied samples, HPTLC is the preferred technique for attempting to isolate and fragment a given ion to obtain a rapid MS/MS spectra record. If capture fails, another replicate can be attempted, unlike LC-MS, where the sample must be re-injected in a new run and eluted again.
Literature
[1] A. Claver et al. Front Plant Sci 15 (2024)1386023.
[2] J.M. Escuín et al. JPC 38 (2025) 455.
Contact: Dr. Vicente L. Cebolla, Instituto de Carboquímica, CSIC, C/ Miguel Luesma Castán, 4.50018 Zaragoza, Spain, vcebolla@icb.csic.es