
HPTLC fingerprinting for distinguishing Kalanchoe species and identifying active secondary metabolites
This research represents a collaborative effort between experts in pharmacognosy, analytical chemistry, and natural products from Brazil and the United States.
Dr. Flávio L. Beltrame is an Associate Professor at the State University of Ponta Grossa (Brazil). He holds a Ph.D. in Organic Chemistry from São Carlos Federal University (Brazil) and completed a postdoctoral fellowship in pharmacology of plant secondary metabolites at the University of Mississippi (USA). His expertise spans ethnopharmacology, natural products chemistry, and pharmaceutical technology, applied to complex matrices.
Dr. Wilmer H. Perera has been the Laboratory Director at CAMAG Scientific Inc. (USA), where he develops and validates HPTLC methodologies, manages analytical projects, and coordinates training courses. He is the Secretary General of the HPTLC Association – North America Chapter, and a member of the Pan-American Expert Panel. With a Ph.D. in Biochemistry and academic training in chemistry and natural products, he has extensive experience in the isolation and identification of bioactive compounds.
Dr. Evelyn A. de Andrade holds a Ph.D. in Pharmaceutical Sciences from the State University of Ponta Grossa (Brazil), with a period at the University of North Carolina Wilmington (UNCW). She is currently a postdoctoral researcher at the Institute of Chemistry of São Carlos (USP, Brazil), and her expertise includes pharmacognosy and natural products chemistry, with a research focus on organic chemistry applied to biological systems.
Together, they applied advanced chromatographic tools to support the quality control and authentication of Kalanchoe species and medicinal plant products.
Introduction
For centuries, medicinal plants have formed the basis for the discovery and development of therapeutic agents, with numerous species remaining extensively used in traditional medicine. The genus Kalanchoe (Crassulaceae), comprising over 170 recognized species distributed across tropical and subtropical regions, possesses a longstanding history of use in complementary and alternative therapies. Frequently referred to as miracle leaf, a variety of Kalanchoe species are employed in folk medicine to address wounds, infections, inflammatory conditions, and other health concerns [1-3].
Despite their cultural and therapeutic significance, the morphological similarity among Kalanchoe species, together with the frequent use of common trivial names, often results in confusion, misidentification, and even adulteration within commercial products [4,5]. Such issues can compromise the efficacy and safety of herbal remedies, requiring the implementation of rigorous quality control strategies. In addition, some effect-directed assays were performed to detect active constituents in the species.
Analytical methodologies that enable precise authentication and chemical characterization are indispensable. HPTLC represents a rapid, reproducible, and cost-effective technique for generating chemical fingerprints from plant extracts, thereby facilitating species authentication and quality assurance. In the present study, fully automated HPTLC PRO analysis was conducted on five Kalanchoe species of medicinal interest: K. crenata, K. daigremontiana, K. marmorata, K. pinnata, and K. × houghtonii. By establishing clear chromatographic profiles, this approach offers reliable tools for differentiating morphologically similar species and aids in the standardization of herbal raw materials derived from Kalanchoe.
Standard solutions
Stock solutions of quercetin, rutin, chlorogenic acid, and kaempferol are prepared at 1 mg/mL each in methanol. Working concentrations: quercetin, kaempferol, and chlorogenic acid at 200 µg/mL, rutin at 400 µg/mL. The Universal HPTLC mix (UHM) is used as a system suitability test (SST).
Sample preparation
Fresh leaf and stem materials from five Kalanchoe species (K. crenata, K. daigremontiana, K. marmorata, K. pinnata, and K. × houghtonii) are extracted with water 1:10 (W/V) using turbo-extraction. The extracts are then diluted to 20 mg/mL in water for HPTLC analysis.
Chromatogram layer
HPTLC plates silica gel 60 F254 (Merck), 20 × 10 cm are used.
Sample application
Samples (10 µL), SST, and standard solutions (2 µL) are applied as bands using the HPTLC PRO Module Application, band length 8.0 mm, distance from the left edge 20.0 mm, track distance 11.4 mm, distance from the lower edge 8.0 mm.
Chromatography
Plates are developed in the HPTLC PRO Module DEVELOPMENT after activation at 33% relative humidity with MgCl2 for 10 min. Conditioning with mobile phase n-butyl acetate – methanol – water –formic acid 7.5:2:1:1 (V/V/V/V) at 25% pump power from 30 to 70 mm developing distance. Plates are dried in the chamber after development.
Post-chromatographic derivatization
Plates are heated to 100° C for 90 s. For the first derivatization, 1.5 mL of NP reagent (1 g of 2-aminoethyl diphenylborinate in 100 mL of methanol) is sprayed using Nozzle 1 (Level 3) in the HPTLC PRO Module DERIVATIZATION. For the second derivatization, 1.8 mL of anisaldehyde reagent (85 mL of ice-cooled methanol mixed with 10 mL of acetic acid and 5 mL of sulfuric acid) is sprayed using Nozzle 2 (Level 2), followed by heating at 100 °C for 90 s.
Three types of antioxidant capacity assays are subsequently performed on the developed plates:
- Folin-Ciocalteau assay – The reagent is diluted 1:10 (V/V) in methanol, and the plate is dipped into the solution using an Immersion device, with the dipping time set to 0 and speed 5. After 30 min, images are taken under white light using the TLC Visualizer 2.
- DPPH• assay – The reagent is prepared at 0.05% (W/V) in methanol, and 3 mL of this solution is sprayed onto the plate with a Derivatizer equipped with a blue nozzle at spraying level 3. The plate was kept in the dark for 30 min, after which images are recorded under white light.
- ABTS+• assay – The reagent is prepared at 0.04% (W/V) in water and subsequently diluted 1:1 (V/V) in methanol. Then, 3 mL of this solution is sprayed onto the plate using the Derivatizer equipped with a yellow nozzle at spraying level 3. White light images are taken after 30 min of derivatization.
Documentation
The images arecaptured using the TLC Visualizer 2 in UV 254 nm, UV 366 nm, and white light.
Results and discussion
The HPTLC fingerprint of the five Kalanchoe species under UV 366 nm after derivatization with NP reagent showed species-specific banding patterns. Differences in band positions and color intensity enabled clear species discrimination. Quercetin and kaempferol derivatives were observed as the main phenolic compounds. Kalanchoe daigremontiana exhibited greenish zones associated with kaempferol glycosides, while K. crenata showed intense yellow bands corresponding to quercetin derivatives.
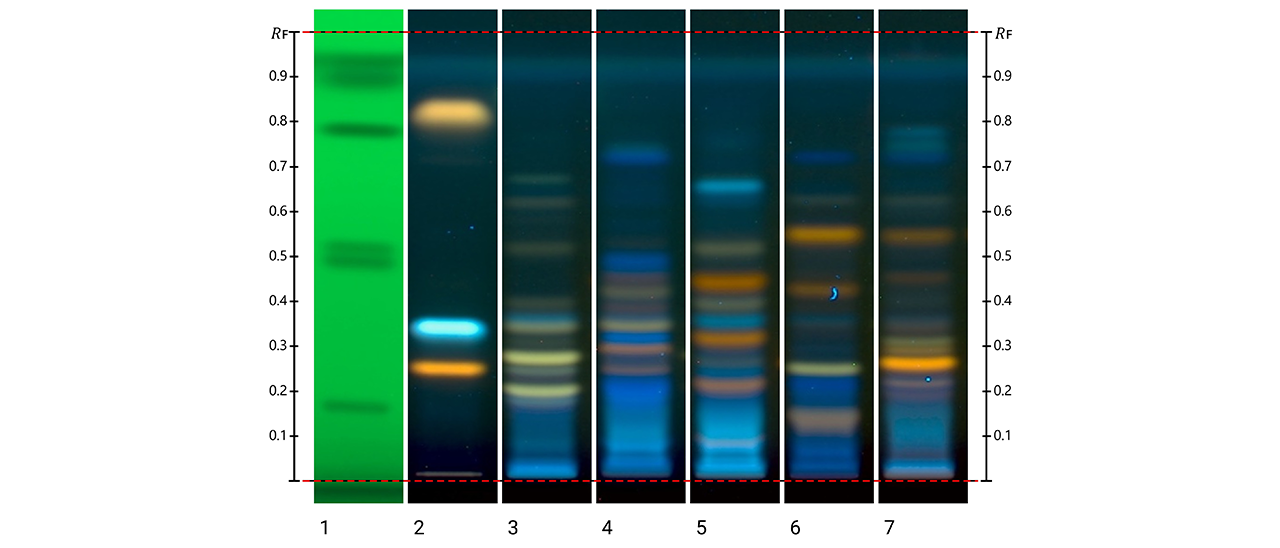
HPTLC fingerprints of Kalanchoe species (NP reagent, UV 366 nm and SST UV 254 nm). Track 1: system suitability test (SST); track 2: rutin, chlorogenic acidand quercetin (with increasing RF); track 3: K. daigremontiana extract; track 4: K. × houghtonii extract; track 5: K. crenata extract; track 6: K. marmorata extract; and track 7: K. pinnata extract.
Post-chromatographic derivatization was also applied to evaluate antioxidant activity directly on the plates. Three complementary assays were performed (Folin-Ciocalteu, DPPH•, and ABTS+•), and revealed clear evidence of antioxidant potential across all species. In the Folin Ciocalteu assay, grayish/bluish zones indicated compounds with reducing capacity; the DPPH• assay revealed zones where the free radical was decoloured, corresponding to strong radical-scavenging compounds. The ABTS+•assay produced decoloration of the ABTS+•, confirming the reducing capacity of specific compounds in the extracts. The reactive zones in all assays corresponded closely to the phenolic bands visualized with NP reagent.
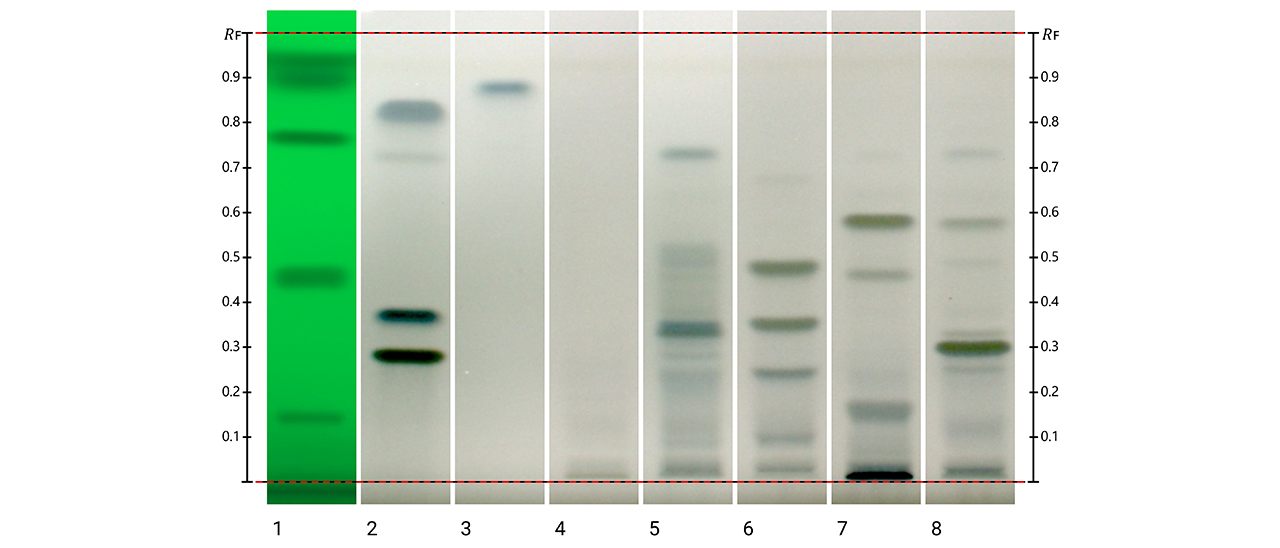
Post-chromatographic Folin–Ciocalteu assay on HPTLC plates under white light. Blue zones correspond to phenolic-rich regions. Track 1: SST (UV 254 nm); track 2: rutin, chlorogenic acid and quercetin (with increasing RF); track 3: kaempferol; track 4: K. daigremontiana extract; track 5: K. × houghtonii extract; track 6: K. crenata extract; track 7: K. marmorata extract; and track 8: K. pinnata extract.
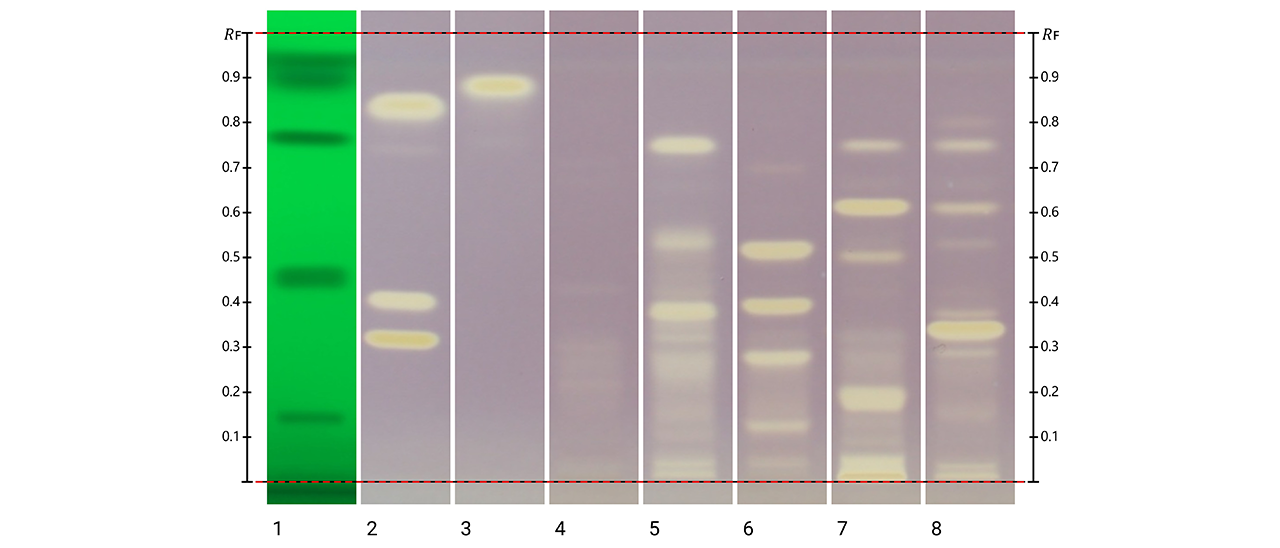
Post-chromatographic antioxidant activity assay of Kalanchoe extracts using DPPH• before imaging under white light. Yellow bands on a purple background indicate radical-scavenging activity. Track 1: SST (UV 254 nm); track 2: rutin, chlorogenic acid and quercetin (with increasing RF); track 3: kaempferol; track 4: K. daigremontiana extract; track 5: K. × houghtonii extract; track 6: K. crenata extract; track 7: K. marmorata extract; and track 8: K. pinnata extract.
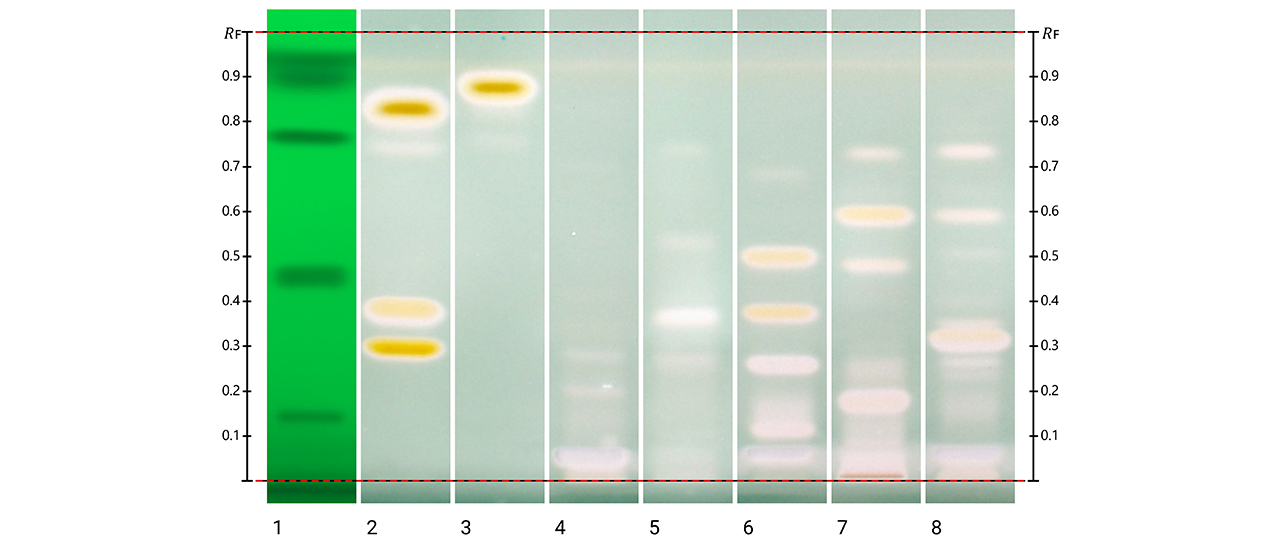
Post-chromatographic antioxidant activity assay of Kalanchoe extracts using ABTS+•, detection under white light. Colorless zones on a bluish-green background indicate antioxidant-active areas corresponding to phenolic compounds. Track 1: SST (UV 254 nm); track 2: rutin, chlorogenic acid and quercetin (with increasing RF); track 3: kaempferol; track 4: K. daigremontiana extract; track 5: K. × houghtonii extract; track 6: K. crenata extract; track 7: K. marmorata extract; and track 8: K. pinnata extract.
The antioxidant active zones detected in the Folin-Ciocalteu, DPPH• and ABTS+• assays are in agreement with the phenolic zones visualized with NP derivatization reagent, indicating that those phenolic compounds likely contribute to the reducing capacity of the extracts.
Overall, the combination of HPTLC fingerprinting and antioxidant assays on the plate provided a robust, reproducible, and highly informative approach for the chemical and functional characterization of Kalanchoe species. These findings confirm the suitability of HPTLC PRO as a robust, reproducible platform for species authentication of Kalanchoe materials.
Literature
[1] M.A. Quazi et al. Int. J. Pharm. Sci. Res. 9 (2018) 1000.
[2] S.V. Pattewar et al. Int. J. Pharm. Sci. Rev. Res. 12 (2012) 20.
[3] N.P. Yadav et al. Int. J. Ayurveda Res. 4 (2003) 152.
[4] G.F. Smith et al. Bothalia 48 (2018) 1.
[5] B. Descoings. Illustr. Handb. Succulent Plants (2003) 144.
[6] S. Sasidharan et al. Evid. Based Complement. Alternat. Med. 8 (2011) 1.
[7] H. Wagner et al. Plant Drug Anal. (1996) 304.
[8] E. Reich et al. High-Perform. Thin-Layer Chromatogr. (2007) 45.
Contact: Dr. Wilmer H. Perera, CAMAG Scientific, Inc., 515 Cornelius Harnett Drive Wilmington, NC 28401, USA, wilmer.perera@camag.com