
Analysis of spirits using HPTLC: general screening and detection of glycerol addition
Introduction
The analysis of spirits involves both chemical and sensory evaluations to ensure product authenticity, quality, and compliance with regulations. Common spirits such as whisky, vodka, rum, tequila, and gin contain ethanol, water, and a diverse range of trace compounds that influence their flavor, aroma, and physical properties.
Advanced analytical techniques are typically employed in spirit analysis. Gas Chromatography (GC) is widely used for volatile compounds, while High-Performance Liquid Chromatography (HPLC) targets non-volatile constituents. In this study, High-Performance Thin-Layer Chromatography (HPTLC) was used as a versatile tool capable of analyzing both volatile and non-volatile compounds.
HPTLC is not only suitable for quality control but also for research and development, including the detection of adulterants, monitoring of aging processes, and comparison of different production batches or brands. Its ability to analyze multiple samples simultaneously, combined with low solvent consumption and ease of use, makes it especially well-suited for industrial applications.
Standard preparation
Glycerol prepared at 0.5 mg/mL in methanol.
Sample preparation
Spirit samples were used without any pre-treatment and directly transferred into vials.
Chromatogram layer
HPTLC plates silica gel 60 F254 (Supelco, Germany), 20 × 10 cm were employed.
Sample application
20.0 µL (for MPDS and HPDS) and 25.0 µL (for LPDS) of sample solutions and 2.0 µL of standard solutions are applied as 6.0 mm bands with the Automatic TLC Sampler 4 (ATS 4).
Chromatography
For general screening, the complementary developing solvent (CDS) approach was applied using three solvents of different polarities [1]:
- low-polarity developing solvent (LPDS): cyclohexane – butyl acetate 88:12 (V/V)
- medium-polarity developing solvent (MPDS): formic acid – cyclopentyl methyl ether, tetrahydrofuran, water 1:40:24:1 (V/V)
- high-polarity developing solvent (HPDS): dichloromethane – ethanol – formic acid – water 16:16:1:4 (V/V)
All developments were performed in the ADC 2, with a 70 mm migration distance. Relative humidity was adjusted to 33% for 10 min. Chamber saturation was maintained for 20 min for MDS and HPDS.
For detecting glycerol, a separate mobile phase using acetone – 25% ammonia solution – toluene – water – 85:1:5:9.5 (V/V) was used. Relative humidity was adjusted to 33% for 10 min, and chamber saturation was maintained for 20 min [2].
Post-chromatographic derivatization
General screening: 3.0 mL of anisaldehyde sulfuric acid reagent was sprayed using the CAMAG Derivatizer (blue nozzle, spraying level 3) and heated at 100°C for 3 min.
Glycerol detection: 3.0 mL of potassium permanganate (KMnO4) reagent was sprayed (yellow nozzle, spraying level 3), and dried in a cold airflow for 4 min.
Documentation
Plates were documented using the CAMAG TLC Visualizer 2 at the following stages:
Before derivatization: UV 254 nm and UV 366 nm.
After derivatization with anisaldehyde sulfuric acid: UV 366 nm with white light reflection-transmission (WRT).
After derivatization with KMnO4 reagent: white light reflection (WR) and white light reflection-transmission (WRT) 30 min after spraying.
Documentation
Densitometric measurements for glycerol detection were performed using the CAMAG TLC Scanner 4 in fluorescence mode at 520 nm (deuterium lamp), with a 5.0 x 0.2 mm slit and 20 mm/s scanning speed.
Results and Discussion
A wide variety of spirits, including cognac, rum, bourbon, vodka, tequila, whisky, and gin, were analyzed.
Before derivatization, samples developed with the CDS systems showed multiple distinct zones, revealing the presence of various classes of compounds. Blue fluorescent zones observed under UV 366 nm (Detection C) seem to be characteristic of barrel-aged spirits such as cognac (tracks 1-2), amber rum (tracks 3-4), bourbon (tracks 5-6), aged tequila (Añejo, reposado; tracks 9-10), and whisky (tracks 11-12). These zones were notably absent in clear spirits like vodka (tracks 7-8) and gin (tracks 13-14), supporting the hypothesis that such fluorescence originates from compounds absorbed during barrel aging, such as lignin-derived congeners.
Interestingly, analysis of two gin samples – one regular (track 13) and one alcohol-free (track 14) – revealed compositional differences. The alcohol-free gin displayed a unique zone under UV 254 nm (Detection B), suggesting the addition of non-volatile components or flavoring agents specific to its formulation.
Numerous spirit samples, including cognac, rum, bourbon, vodka, tequila, whisky, and gin, were collected, and tested.
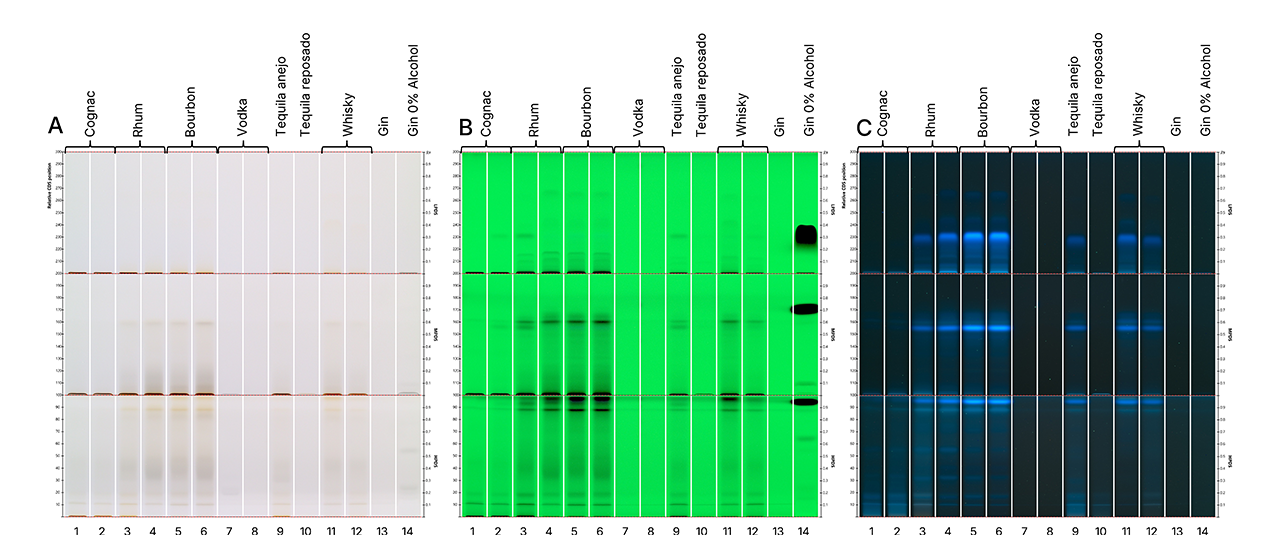
HPTLC fingerprints of tested spirit samples in white light (A), UV 254 nm (B), and UV 366 nm (C) prior to derivatization.
Post-chromatographic derivatization with anisaldehyde reagent (Detection D) significantly enhanced the zone visibility. Brown-colored zones below RF 0.3, particularly in HPDS-developed plates, were observed across all samples – including vodka and gin– indicating the possible presence of carbohydrates such as glucose or added sugars. Their presence in clear spirits, which are not typically sweetened, raises questions about additives or flavor enhancers.
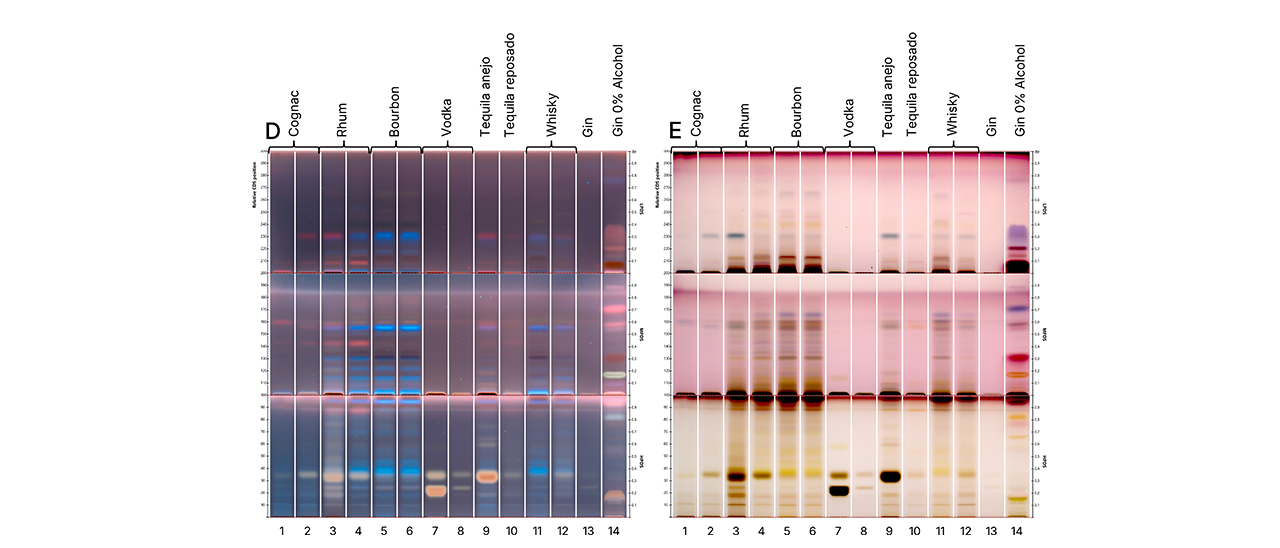
HPTLC fingerprints of tested spirit samples in UV 366 nm (D), and white light (E) after derivatization.
For glycerol detection, the specific developing system followed by KMnO4 derivatization enabled the identification of glycerol-related zones. These zones fluoresced under UV 366 nm in white light reflection mode (WRT), and densitometric analysis confirmed their presence based on retention factor (RF) and fluorescence profile. Spirits known to contain added glycerol to enhance mouthfeel – such as certain flavored vodkas and specialty liqueurs – showed distinct signals corresponding to glycerol. This method is particularly valuable, as glycerol (E422) is permitted as a food additive in alcoholic beverages within the EU and Switzerland, but its use must comply with specific regulatory limits [3].
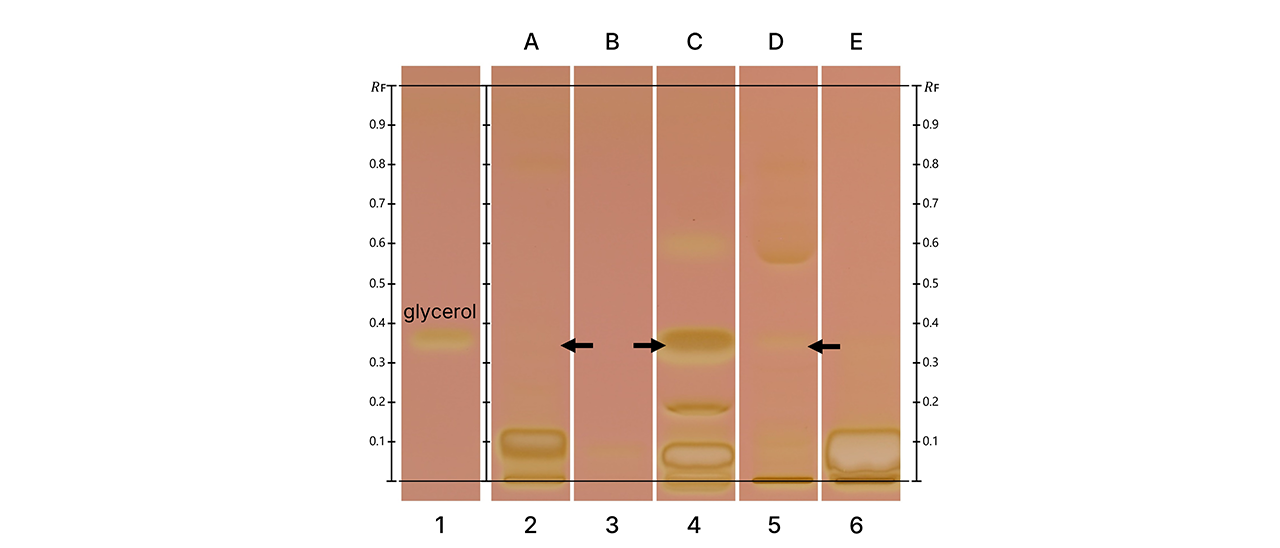
HPTLC fingerprints of tested five different vodka samples (A-E) in white light after derivatization using the glycerol method.
Conclusion
This study highlights the strong potential of HPTLC as a practical and powerful analytical technique for the food and beverage industry. In the context of spirits, it offers clear advantages: the ability to analyze multiple samples simultaneously, detect a broad range of compounds – from natural aging markers to added substances like sugars and glycerol – and deliver results quickly with minimal sample preparation.
The successful application of HPTLC to detect glycerol and differentiate between various spirit types underscores its reliability and versatility. It is particularly well-suited for routine screening, quality control, and regulatory compliance, where rapid and reproducible results are essential.
Literature
[1] CBS 129: HPTLC routine analysis using complementary developing solvents.
[2] CBS 134: Detection and limit test of diethylene glycol and ethylene glycol impurities in syrup by HPTLC.
[3] https://www.efsa.europa.eu/en/efsajournal/pub/4720 (accessed on 23/07/2025)
Contact:
Robert Gibson, Sazerac Switzerland GmbH, 6005 Lucerne, Switzerland, rgibson@sazerac.com
Tiên Do, CAMAG, 4132 Muttenz, Switzerland, tien.do@camag.com