HPTLC fingerprint profiling for determination of bioactive ingredients in Indian propolis

Introduction
To identify the propolis type, a simplified, rapid, low-cost, low-environmental impact, and easily adoptable analytical methodology was developed, extending to the standardization of selected neuroprotective components in Indian propolis. The versatility of HPTLC, with various derivatizing reagents and orthogonal detection capabilities, allows for increased applications. With the advent of thin-layer chromatography-effect directed analysis, it enables direct screening on the TLC plate, establishing preliminary evidence of the biological activities. Thus, this HPTLC method is valuable for rapid chemical profiling and simultaneous screening of antioxidant and anticholinesterase activities of Indian propolis. Also, educating beekeepers about its medicinal value can help them generate additional revenue.
Standard solutions
Sample preparation
Chromatogram layer
HPTLC plates silica gel 60 F254 (Merck), 20 x 10 cm are used.
Sample application
1.0-10.0 μL of standard solutions (7-point calibration) and 2.0 and 5.0 μL of sample solutions are applied as bands with the Linomat 5 (with N2). Plate layout: 15 tracks, band length 6.0 mm, distance from left plate edge 15.0 mm, track distance 11.4 mm, distance from the lower edge 8.0 mm.
Chromatography
Plates are developed in the twin-trough chamber with chamber saturation for 30 min (with filter paper) and development with toluene ‒ ethyl acetate ‒ formic acid 74:26:5 (V/V) to the migration distance of 80 mm (from the lower edge), followed by drying for 5 min.
Post-chromatographic derivatization
Note: The derivatization was conducted on three different developed plates.
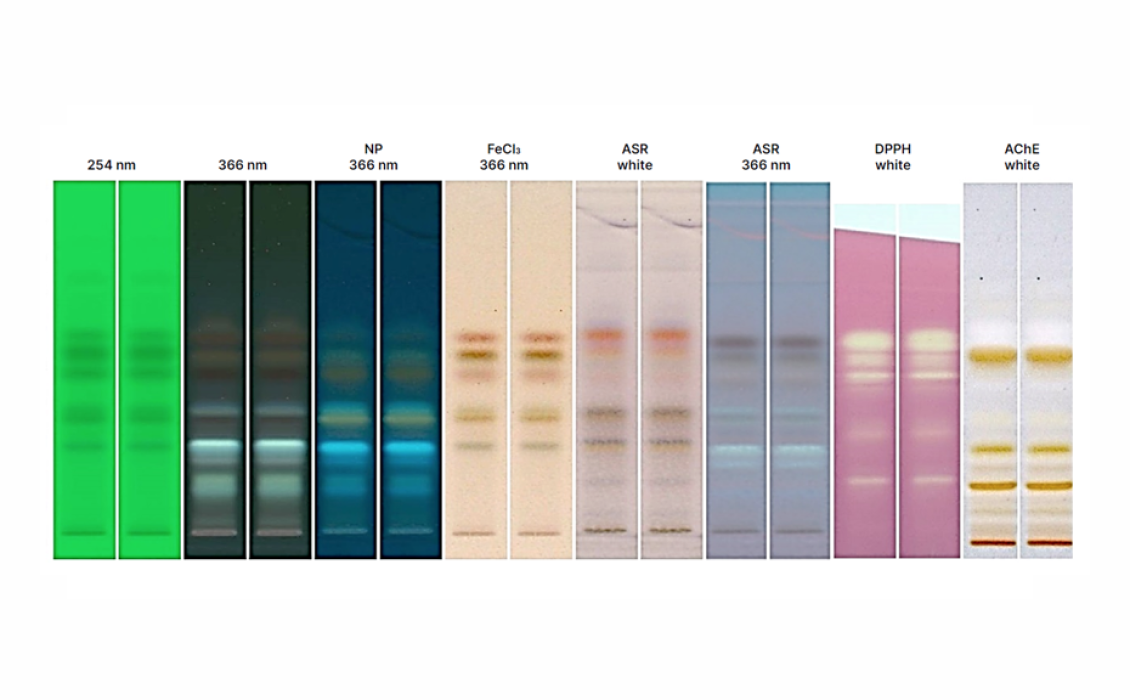
Post-chromatographic bioautography
The developed plate is immersed into a 2,2-diphenyl-1-picryl hydrazyl solution (DPPH – 0.25 % (W/V) in methanol), stored in the dark for 30 min. The yellow zones captured against purple background are an indicator of antioxidant components when visualized in white light. The Ellman assay protocol was used wherein the developed plate is first immersed in a solution of 5,5′-dithiobis-2-nitrobenzoic acid (DTNB) and acetylthiocholine iodide (ATCI) (1 mM DTNB and 1 mM ATCI in buffer A) until the plate was saturated, dried for 5 min and then around 3-4 mL of acetylcholinesterase enzyme solution (Electrophorus electricus – AChE – 3 U/mL) is sprayed onto the plate. The white band on the plate is an indicator of acetylcholinesterase inhibition.
Documentation
Images of the plate are captured with the TLC Visualizer 2 in UV 254 nm, UV 366 nm, and white light.
Densitometry
Mass spectrometry
Results and discussion
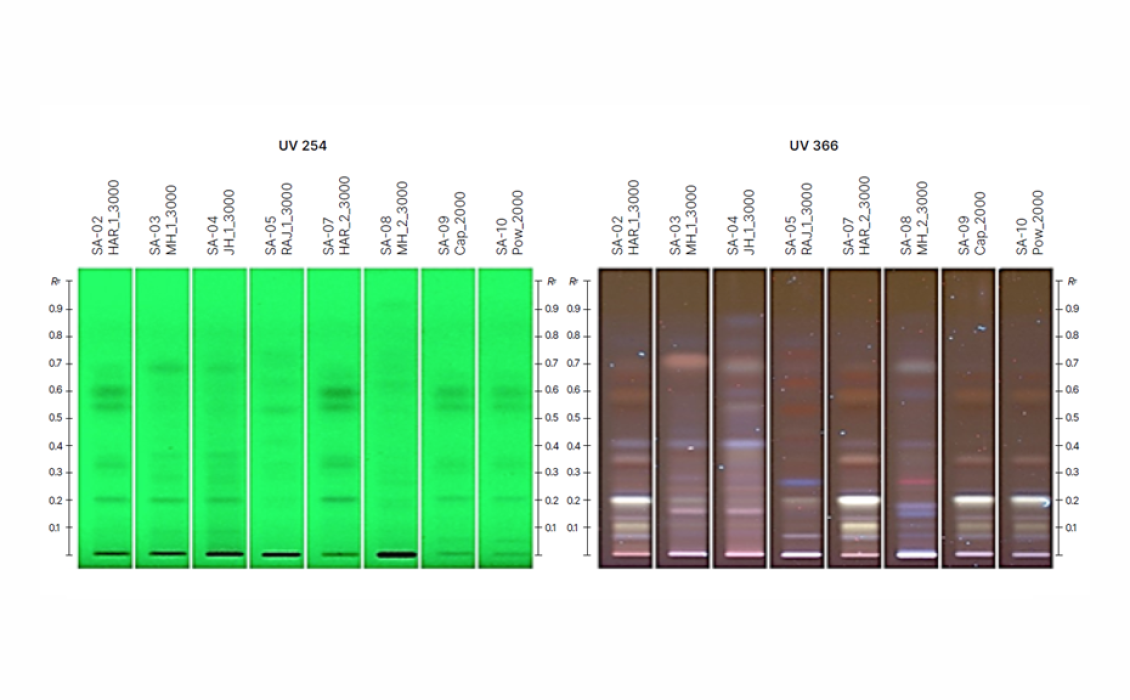
The HPTLC fingerprint image of the various propolis extracts is shown, and the profiles are key indicators of the diversity in vegetation across different regions. The sample coded HAR was mainly of ‘O-type’ propolis due to the presence of flavonoids like chrysin, galangin, pinocembrin, as well as non-flavonoids like p-coumaric acid, matching the characteristic bands of the standard when derivatized with various reagents. Interestingly, the applicability of the method on two marketed products presented a similar fingerprint to that of the HAR extract.
The optimized method is found to be precise (%RSD ≤ 2.0 %), accurate (90‒110 %), linear over the concentration ranges (r2 ≥ 0.995), sensitive and robust resulting in the RF values of 0.235, 0.353, 0.552, 0.606, and 0.655 for luteolin, p-coumaric acid, chrysin, galangin, and pinocembrin, respectively. Pinocembrin (2.30 ± 0.12 % W/W) and galangin (5.78 ± 0.30 % W/W) are found in the highest concentrations in the HAR sample. The m/z values of the molecular ion and fragment ions from the isolated sample bands matched those of the standards, further confirming the identity of the peaks. The bands with RF values corresponding to chrysin, galangin, and pinocembrin showed strong antioxidant activity, as indicated by bright yellow zones against a purple background, while the white bands in the extract fingerprint that appeared along the plate following the Ellman’s assay are indicative of acetylcholinesterase inhibitors.
Thus, the developed analytical method with orthogonal capabilities can be universally applied to different propolis extracts and formulated propolis products as a quick screening method for fingerprint and neuroprotective profiling.
SMOOTH & PRECISE OPERATION
[1] Sankaran, S. et al. (2024) J Planar Chromat 37 (3), 233–245
[2] Bankova, V. et al. (2019) J Apic Res 58, 1–49
[3] Sankaran, S. et al. (2023) J Biol Active Prod Nat 13, 76–93.
Further information is available in the article published “Sustainable instrumental thin-layer chromatography-based methodology for standardization of neuroprotective components in propolis collected from India” J Planar Chromat 37, 233–245 (2024). https://doi.org/10.1007/s00764-024-00307-x or on request from the authors.
Contact: Sandeep Sankaran, Department of Quality Assurance Techniques, Poona College of Pharmacy, Bharati Vidyapeeth (Deemed to be) University, Pune, Maharashtra 411038, India, sandeepsss1992@gmail.com