Comprehensive HPTLC fingerprinting for the quality control of Angelica gigas root

Introduction
The required conditions for an efficient purification (ca. 75% use silica gel at Oril Industrie) are determined by TLC. Then, the purification progress is checked by preparative column chromatography via HPTLC. Twenty fractions were analyzable within 1 hour. TLC/HPTLC is the method of choice due to its simplicity, rapidness and the successful scale up from TLC to preparative separations. HPTLC-MS helped to quickly resolve the composition of a mixture.
Sample preparation
Chromatogram layer
TLC plate silica gel 60 F254 (Merck), 20 x 5 cm HPTLC plate silica gel 60 F254s (Merck), 20 x 10 cm
Sample application
Chromatography
Documentation
Mass spectrometry
NMR
Results and discussion
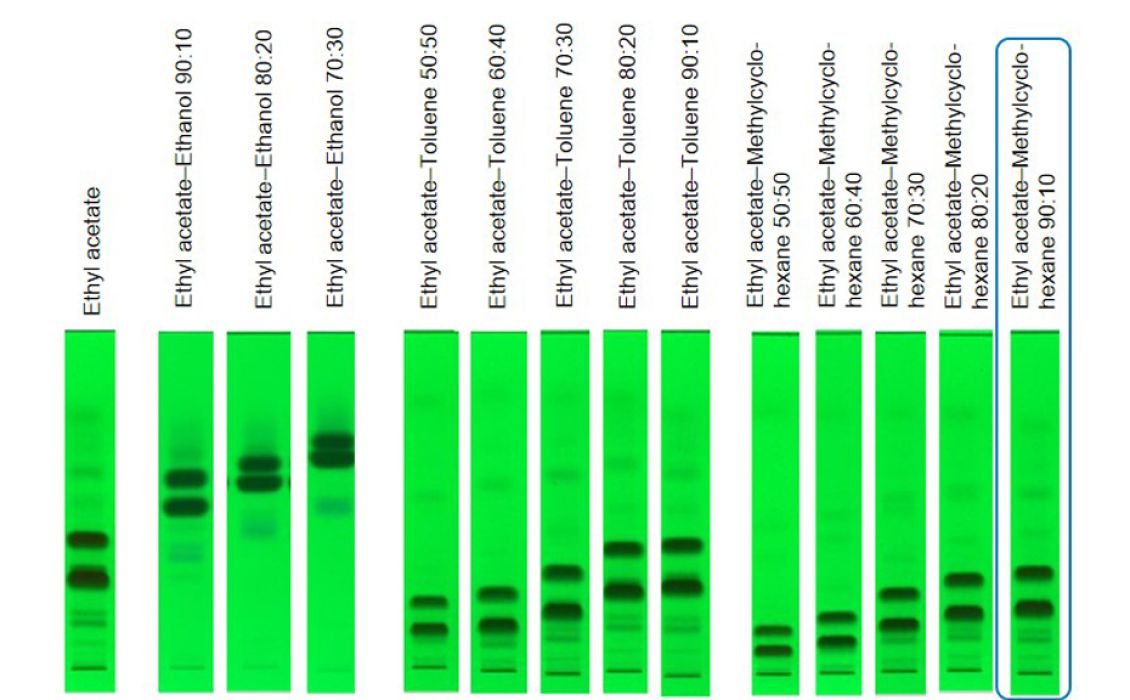
The aim of this study was to obtain a batch with a purity >99% of the isomer Z, containing <1% of isomer E and < 0.15% of other impurities. By RP-HPLC the isomers were not separated satisfyingly, and TLC was selected for method development to separate the two isomers. Ethyl acetate – methylcyclohexane 9:1 was the best option to separate all compounds at reasonable hRF values allowing a fast purification.
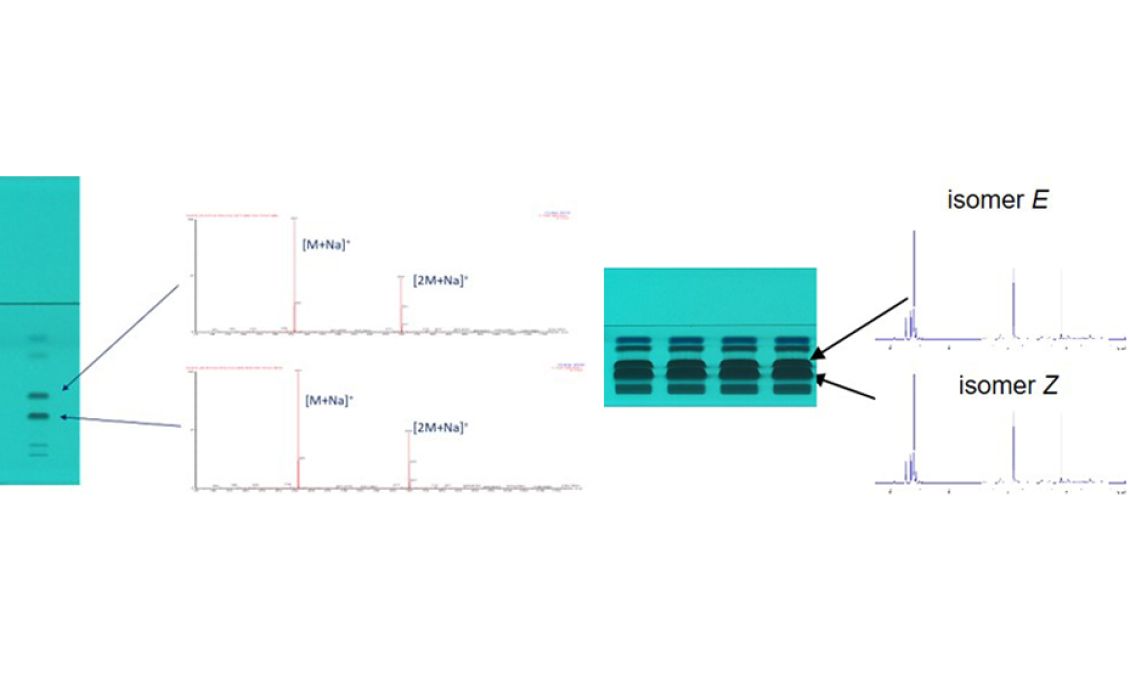
Mass spectra were recorded to characterize the different compounds. The same sodium adduct [M+Na]+ and respective dimer [2M+Na]+ were obtained for both Z/E isomeres. For NMR, the crude product solution was concentrated by a factor of 10, applied (15 μL) on the HPTLC plate, separated, and four zones of each target zone were eluted and combined.
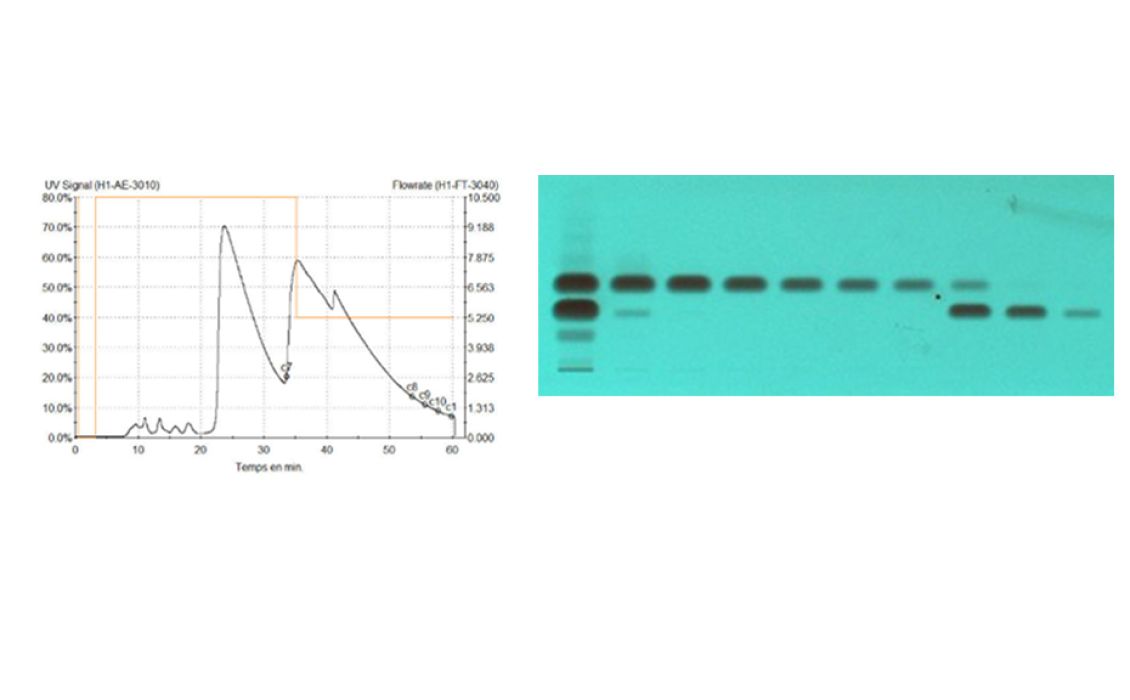
The purification of the crude product (2 kg dissolved in toluene) on a 45-cm column (packed at 40 bars with 40 kg silica gel 60, 15–40 μm, Merck) at a flow rate of 10 L/min with ethyl acetate – methylcyclohexane 9:1 led to a productivity of 20 kg per day. The elution process was monitored online by UV 254 nm detection and in parallel offline by HPTLC.
The different fractions of the target isomer Z were collected and the combined fractions analyzed by NMR. The purity was not sufficient, as the NMR spectrum showed several impurities. Thus, the purification was optimized. The new eluent of dichloromethane – ethanol 19:1 together with a crude product load of 0.5 kg also led to a productivity of 20 kg per day. The purity obtained for the Z isomer was 99.8% with a yield of 88%.
TLC is the best method for development and optimization of purification processes using silica gel. It is simple and allows a rapid upscaling to preparative columns. HPTLC is an efficient tool for offline monitoring of the eluted fractions. Up to 20 fractions can be analyzed in parallel and compared in the HPTLC chromatogram at UV 254 nm, achieving a good overview on the purity and amount of the target compound per fraction.
SMOOTH & PRECISE OPERATION
01
TLC chromatograms at UV 254 nm of the crude product separated with different mobile phases
02
HPTLC chromatograms at UV 254 nm of the crude product (10 g/L, 1 μL versus 100 g/L,15 μL) and mass spectra (left) versus 1H NMR spectra of isomeres (right)
03
Online monitoring of the purification process by LC-UV (254 nm, left) versus offline by HPTLC-UV (individual fractions at 254 nm, right)
Contact: Amélie Havard, Daniel Dron, Oril Industrie (Servier), Industrial Research Centre, Department of Analytical Innovative Technologies R&D, 13 rue Auguste Desgenétais, CS 60125, 76210 Bolbec, France amelie.havard@servier.com, daniel.dron@servier.com